Method for efficiently inducing antibody to hepatitis virus, antibody and detection system
A hepatitis B virus and antibody technology, applied in the direction of virus/phage, virus antigen component, virus, etc., can solve the problem of unable to prevent HBV infection, and achieve the effect of inhibiting infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0150] [Preparation of Antibody]
[0151] The monoclonal antibody of the present invention can be produced, for example, by methods known to those skilled in the art (hybridoma method, phage display method, etc.) described below.
[0152] Antibody-producing B cells can be obtained from the spleen, lymph nodes or peripheral blood of antigen-immunized mice, and antibodies can also be obtained from immunized mice by preparing hybridomas or obtaining cDNA.
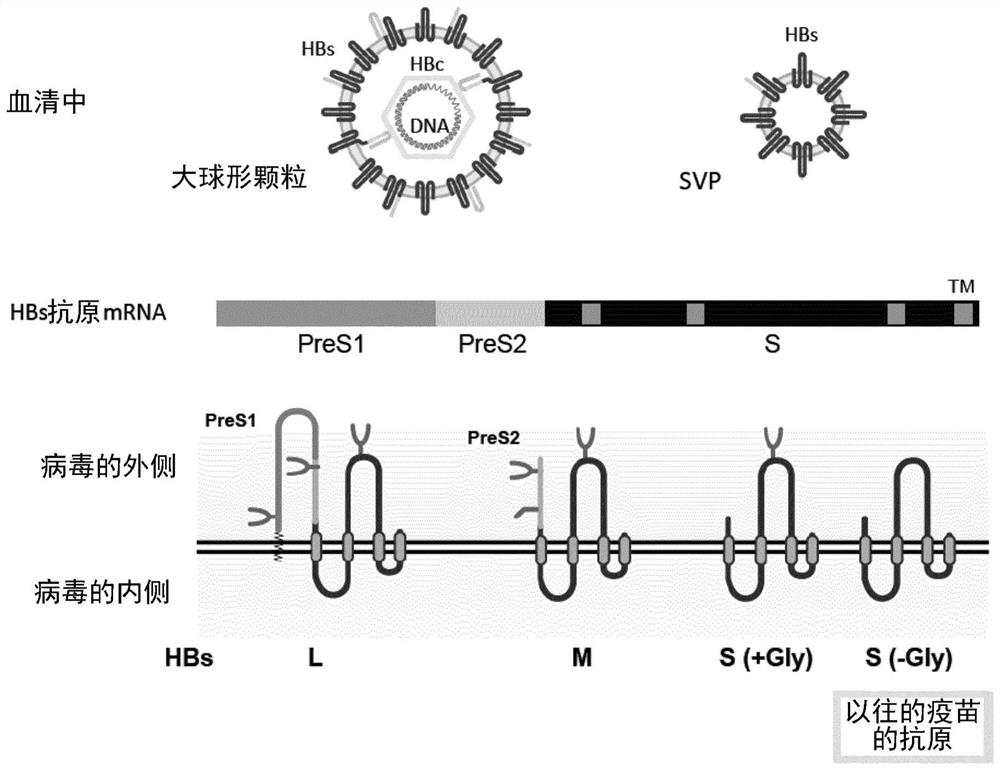
[0153] Specifically, for the hepatitis B virus antigen having sugar chains on the HBs glycoprotein (PreS1, PreS2 or S region), the HBs glycoprotein having the structure of the above formula [1], [2] or [3] The monoclonal antibody to the hepatitis B virus antigen or the hepatitis B virus antigen described in the item "HBs glycoprotein" can be administered to a mammal by Hepatitis B virus antigen, a hepatitis B virus antigen derived from HBs glycoprotein having the structure of the above formula [1], [2] or [3], or the hepatiti...
Embodiment
[0213] Hereinafter, the present invention will be described more specifically by showing examples, but the present invention is not limited to these examples.
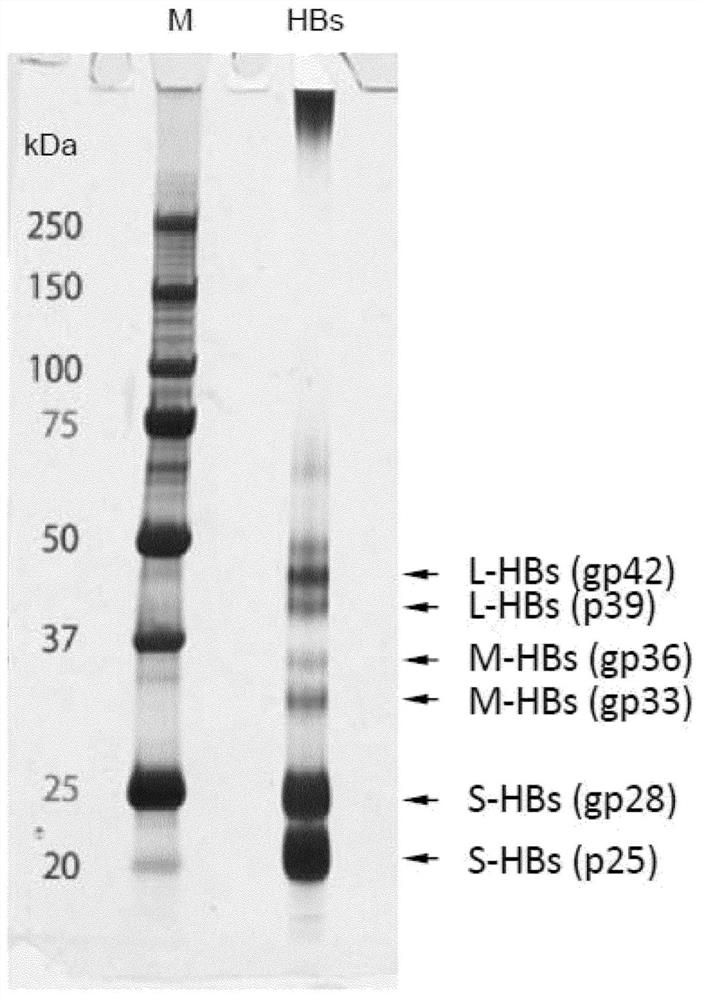
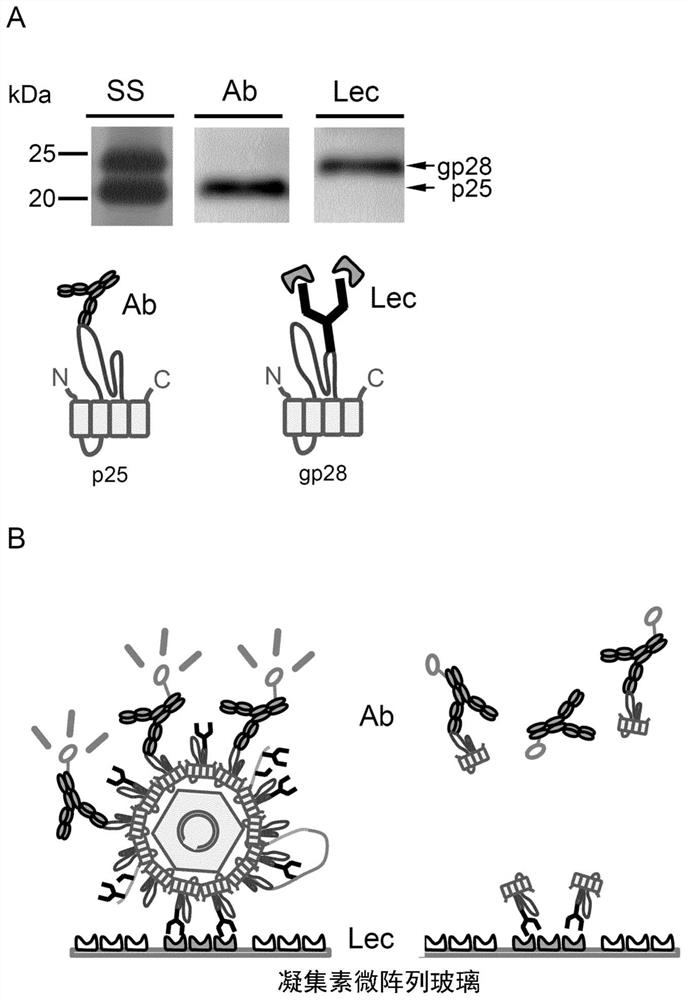
[0214] In order to distinguish large spherical particles from SVP, we first focused on the sugar chain modification on HBV particles, and analyzed the sugar chain attachment and sugar chain structure of each HBs antigen. A particle fraction containing HBV particles was prepared from pooled sera of HBV-infected patients using the ultracentrifugal concentration method. Particle components were used in various studies after inactivation by heat treatment at 60 °C overnight. In the presence of 0.4% SDS and a reducing agent (0.2M dithiothreitol; DTT), the concentrated HBV particles were heat-treated at 95°C for 5 minutes, and analyzed by SDS-PAGE (Fujifilm Wako Pure Chemical Industries, Ltd. Wako Pure Chemical Industries, Ltd.) SuperSep TM Ace, 10-20%) developed and fixed for silver staining ( figure 2 ).
[0215] Sim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com