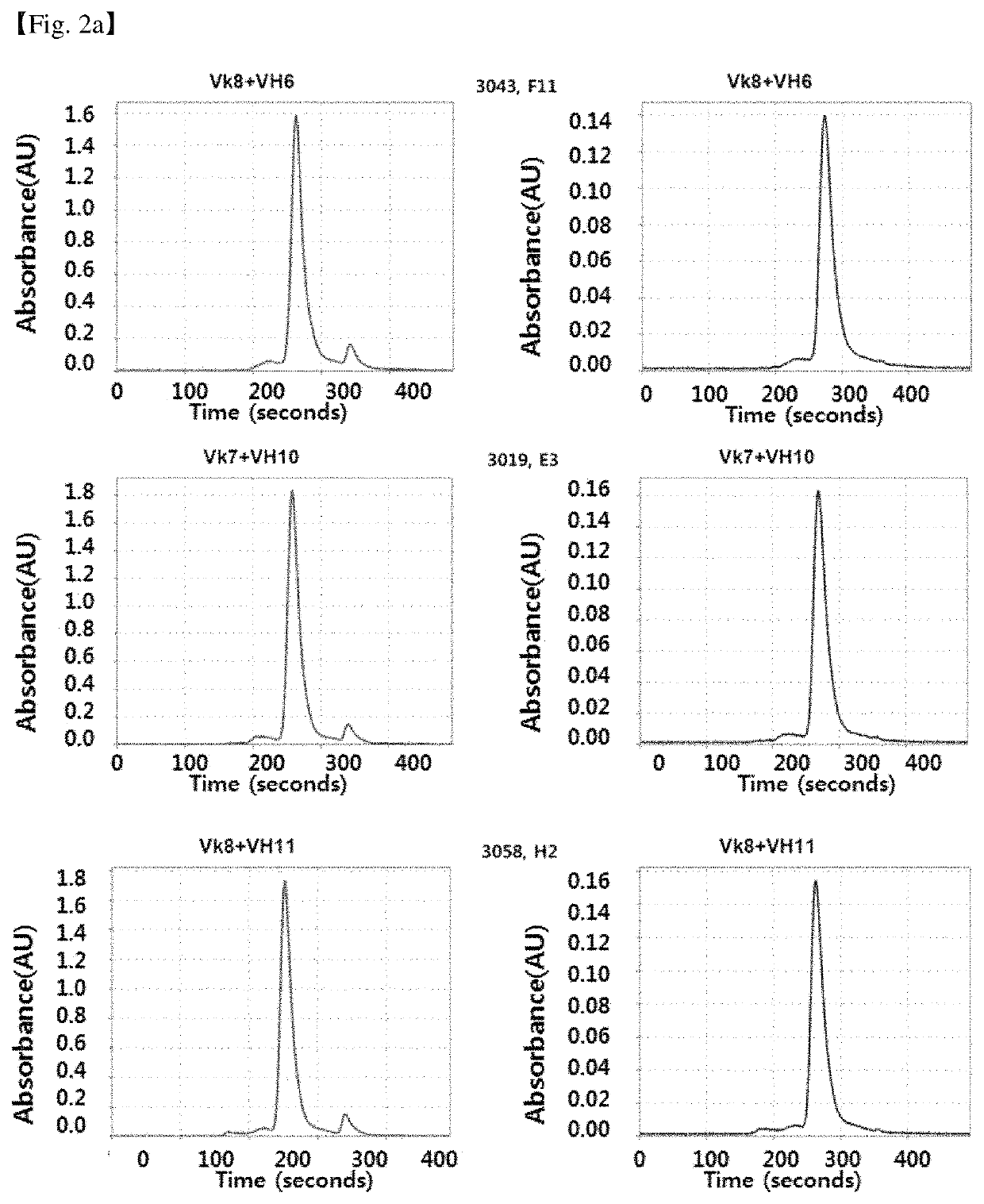
Patents
Literature
34 results about "Antibody induction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody induction therapy is frequently used as an adjunct to the maintenance immunosuppression in adult kidney transplant recipients. Published data support antibody induction in patients with immunologic risk to reduce the incidence of acute rejection (AR) and graft loss from rejection.
Powerful vaccine composition comprising lipopeptide and poly I:C as an adjuvant
ActiveUS8216595B2Stimulate immune responseStrong immune responseSsRNA viruses negative-sensePeptide/protein ingredientsAdjuvantVirus
Owner:CHA VACCINE RES INST CO LTD
Powerful vaccine composition comprising lipopeptide and poly I:C as an adjuvant
ActiveUS20090155308A1Stimulate immune responseStrong immune responseSsRNA viruses negative-sensePeptide/protein ingredientsAdjuvantVirus
The present invention relates to an adjuvant comprising a lipopeptide and poly I:C. When the adjuvant of the present invention is used, the level of antigen specific antibody induction is synergistically increased and Th1 type immune response is also induced. Therefore, the adjuvant of the present invention can be very effectively used as an adjuvant in the formulation of preventive and therapeutic vaccines for viral or parasitic infection and cancer.
Owner:CHA VACCINE RES INST CO LTD
Protein chip of directional immobilizing antibody, its preparing method and use thereof
InactiveCN1402007AReduced activityOvercome the disadvantage of decreased biological activityBiological testingProtein insertionProtein chip
A protein chip with directional fixed antibody for immunodetection medium screening, etc is composed of solid substrate, protein A film, antibody induction film,and hydrophobic polarized layer. Its preparing process includes choosing and cleaning solid substrate, preparing hydrophobic polarized layer, preparing protein A film, and preparing antibody induction film.
Owner:INST OF MECHANICS - CHINESE ACAD OF SCI
Reagents and Methods for Engaging Unique Clonotypic Lymphocyte Receptors
InactiveUS20170261497A1Antibacterial agentsAntibody mimetics/scaffoldsAntiendomysial antibodiesReceptor
Platforms comprising at least one lymphocyte affecting molecule and at least one molecular complex that, when bound to an antigen, engages a unique clonotypic lymphocyte receptor can be used to induce and expand therapeutically useful numbers of specific lymphocyte populations. Antigen presenting platforms comprising a T cell affecting molecule and an antigen presenting complex can induce and expand antigen-specific T cells in the presence of relevant peptides, providing reproducible and economical methods for generating therapeutic numbers of such cells. Antibody inducing platforms comprising a B cell affecting molecule and a molecular complex that engages MHC-antigen complexes on a B cell surface can be used to induce and expand B cells that produce antibodies with particular specificities.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Adeno-associated virus mediated novel coronavirus antibody inducer and vaccine composition
ActiveCN111996216AMild immune responseNo obvious side effectsSsRNA viruses positive-senseViral antigen ingredientsCoronavirus antibodyInducer
The invention provides a novel coronavirus antibody inducer based on an adeno-associated virus. Antibodies in accordance with a novel coronavirus can be generated in bodies through induction, and theinvention provides a virus vector and a vaccine composition including the antibody inducer.
Owner:UNIV OF SCI & TECH OF CHINA
N-linked glycosylation alteration in E0 and E2 glycoprotein of classical swine fever virus and novel classical swine fever virus vaccine
InactiveUS20100104597A1Slow onsetDelaying severitySsRNA viruses positive-senseSugar derivativesVirulent characteristicsWild type
E2 is one of the three envelope glycoproteins of Classical Swine Fever Virus (CSFV). E2 is involved in several functions including virus attachment and entry to target cells, production of antibodies, induction of protective immune response in swine, and virulence. Seven putative glycosylation sites in E2 were modified by site directed mutagenesis of a CSFV Brescia infectious clone (BICv). A panel of virus mutants was obtained and used to investigate whether the removal of putative glycosylation sites in the E2 glycoprotein would affect viral virulence / pathogenesis in swine. We observed that rescue of viable virus was completely impaired by removal of all putative glycosylation sites in E2, but restored when mutation N185A reverted to wild-type asparagine produced viable virus that was attenuated in swine. Single mutations of each of the E2 glycosylation sites showed that amino acid N116 (N1v virus) was responsible for BICv attenuation. N1v efficiently protected swine from challenge with virulent BICv at 3 and 28 days post-infection suggesting that glycosylation of E2 could be modified for development of CSF live-attenuated vaccines. Additionally, a new developed virus, contained deletions of putative glycosylation sites N1 in E2 and N1 in E0 (6b), called N1E0 / 2v, induce a solid protection against the challenge at 3 and 28 days post-inoculation.
Owner:BORCA MANUEL +1
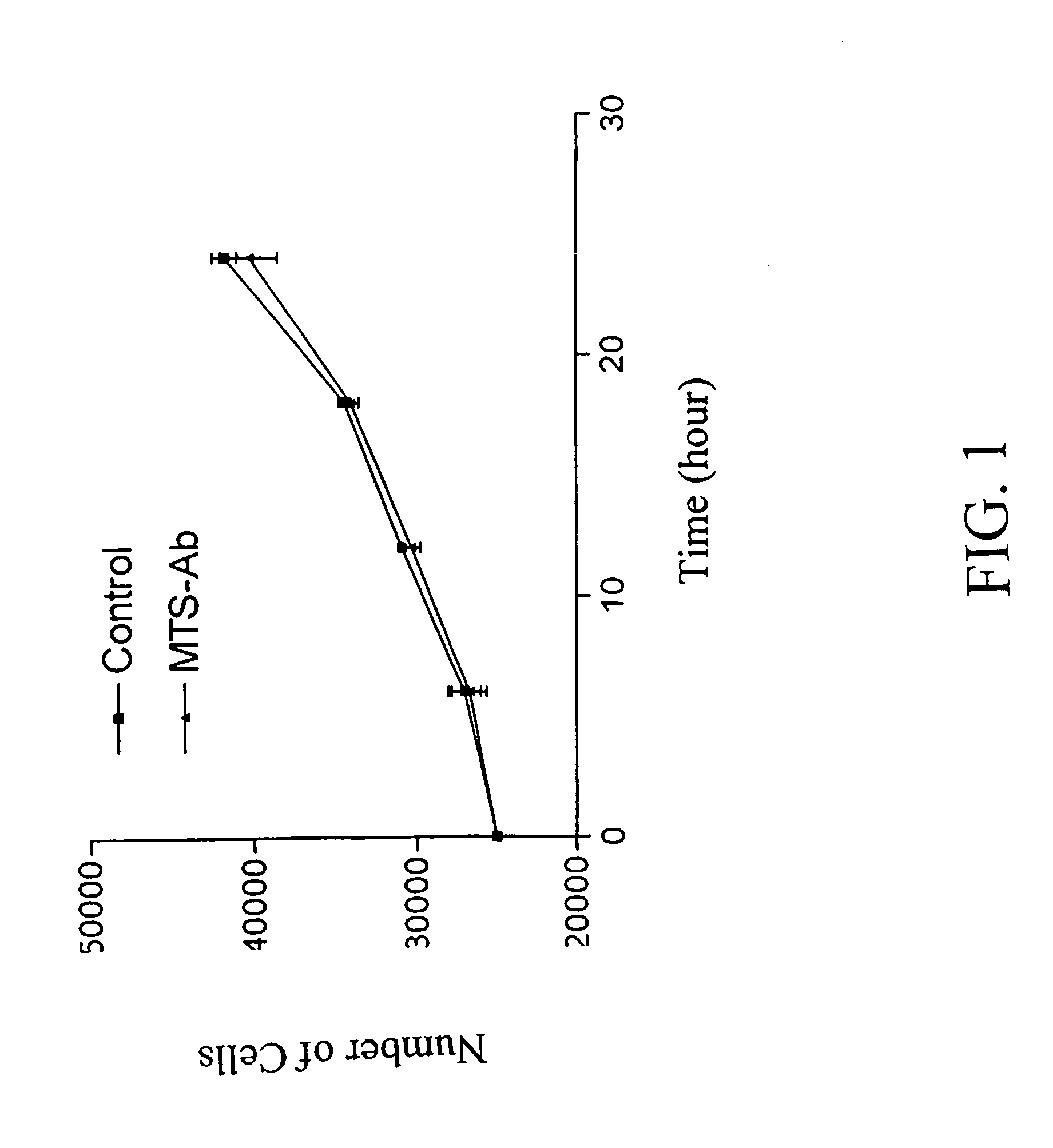
Trans-membrane-antibody induced inhibition of apoptosis
InactiveUS20050033033A1Inhibit apoptosisEffective treatmentHybrid immunoglobulinsNervous disorderAntibody conjugateMembrane Transporters
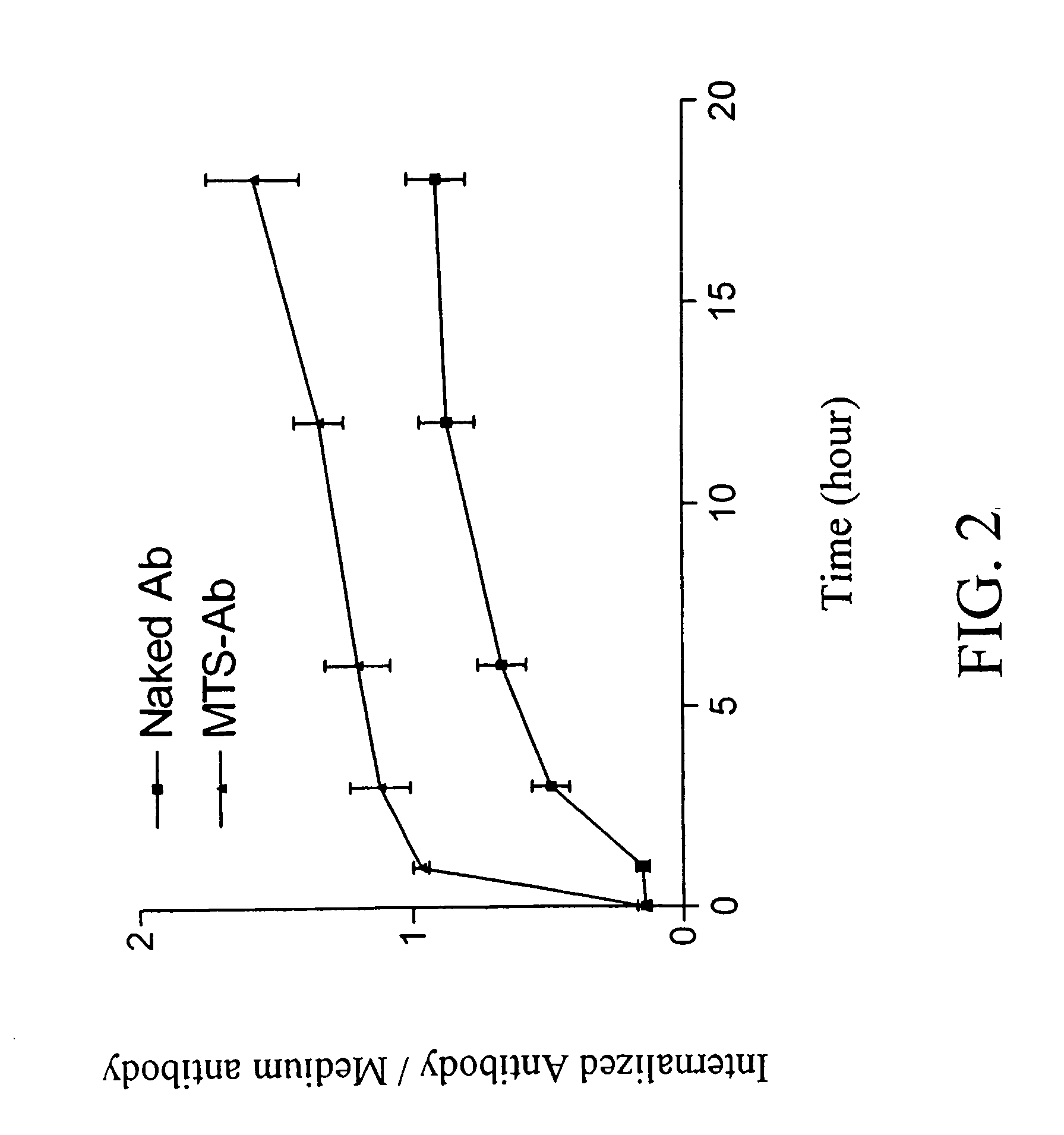
Cell suicide (apoptosis) is associated with pathogenesis, for example, it is the major cause for the loss of neurons in Alzheimer's disease. Caspase-3 is critically involved in the pathway of apoptosis. Superantibody (SAT)-trans-membrane technology has been used to produce antibodies against the caspase enzyme in an effort to inhibit apoptosis in living cells. The advantage of using trans-membrane antibodies as apoptosis inhibitors is their specific target recognition in the cell and their lower toxicity compared to conventional apoptosis inhibitors. It is shown that a MTS-transport-peptide modified monoclonal anti-caspase-3 antibody reduces actinomycin D-induced apoptosis and cleavage of spectrin in living cells. These results indicate that antibodies conjugated to a membrane transporter peptide have a therapeutic potential to inhibit apoptosis in a variety of diseases.
Owner:INNEXUS BIOTECHNOLOGY INT LTD
Vaccine for HPV infection and/or hepatitis b comprising HPV/hbs chimeric protein as active ingredient
InactiveUS20150344529A1Improve productivityQuality improvementBacteriaAntibody mimetics/scaffoldsAntiendomysial antibodiesImmunocompetence
A chimeric protein of HPV-L2 peptide and HBs protein wherein the HPV-L2 peptide is (1) a peptide consisting of the core sequence region of 20 amino acid residues, (2) a peptide inside the core sequence region comprising 6 amino acid residues Gly-Gly-Leu-Gly-Ile-Gly or (3) a peptide consisting of 70 or less amino acid residues obtained by adding an amino acid sequence derived from HPV L2 protein at the N-terminal and / or the C-terminal of the core sequence region; and a vaccine for HPV infection and / or hepatitis B comprising the chimeric protein as an active ingredient. A vaccine comprising the chimeric protein of the present invention is the one that has an increased expression level in a host, an enhanced immune ability (early antibody induction) and a broader spectrum effective for a number of HPV types.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST +1
HIV (human immunodeficiency virus) immunogen and preparation method thereof
InactiveCN102558314ACan't overcomeIn the virus after overcoming the mutationAntiviralsDepsipeptidesAdjuvantHuman immunodeficiency
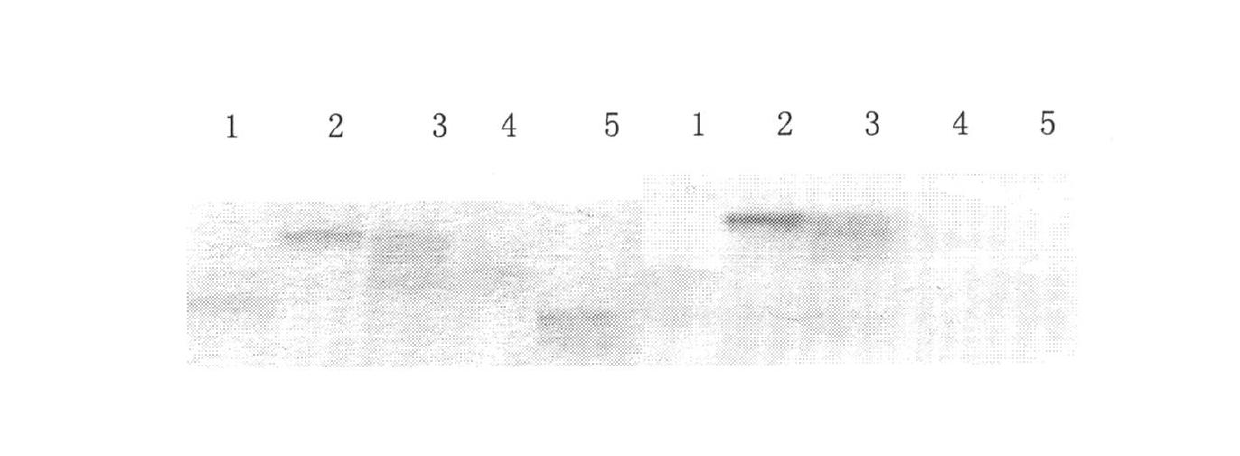
The present invention relates to an HIV (human immunodeficiency virus) immunogen that stimulates broad-spectrum neutralizing antibodies and a vaccine prepared from the HIV immunogen. The HIV immunogen contains a peptide sequence which comprises HIV 4E10 and / or 2F5 and / or Z13 epitope sequences. The immunogen of this invention can stimulate HIV-specific broad-spectrum neutralizing antibodies, and overcomes the delaying problem of antibody induction due to high variability of HIV in the prior art. Animal immune experiments show that the peptide immunogen of the invention supplemented with adjuvants can induce production of specific antibodies in sera of immunized animals, and the induced antibodies has the capacity of broadly neutralizing representative HIV infected cells, and has the potential for development of clinical vaccines against HIV and auxiliary enhancement of HIV vaccine.
Owner:JILIN UNIV
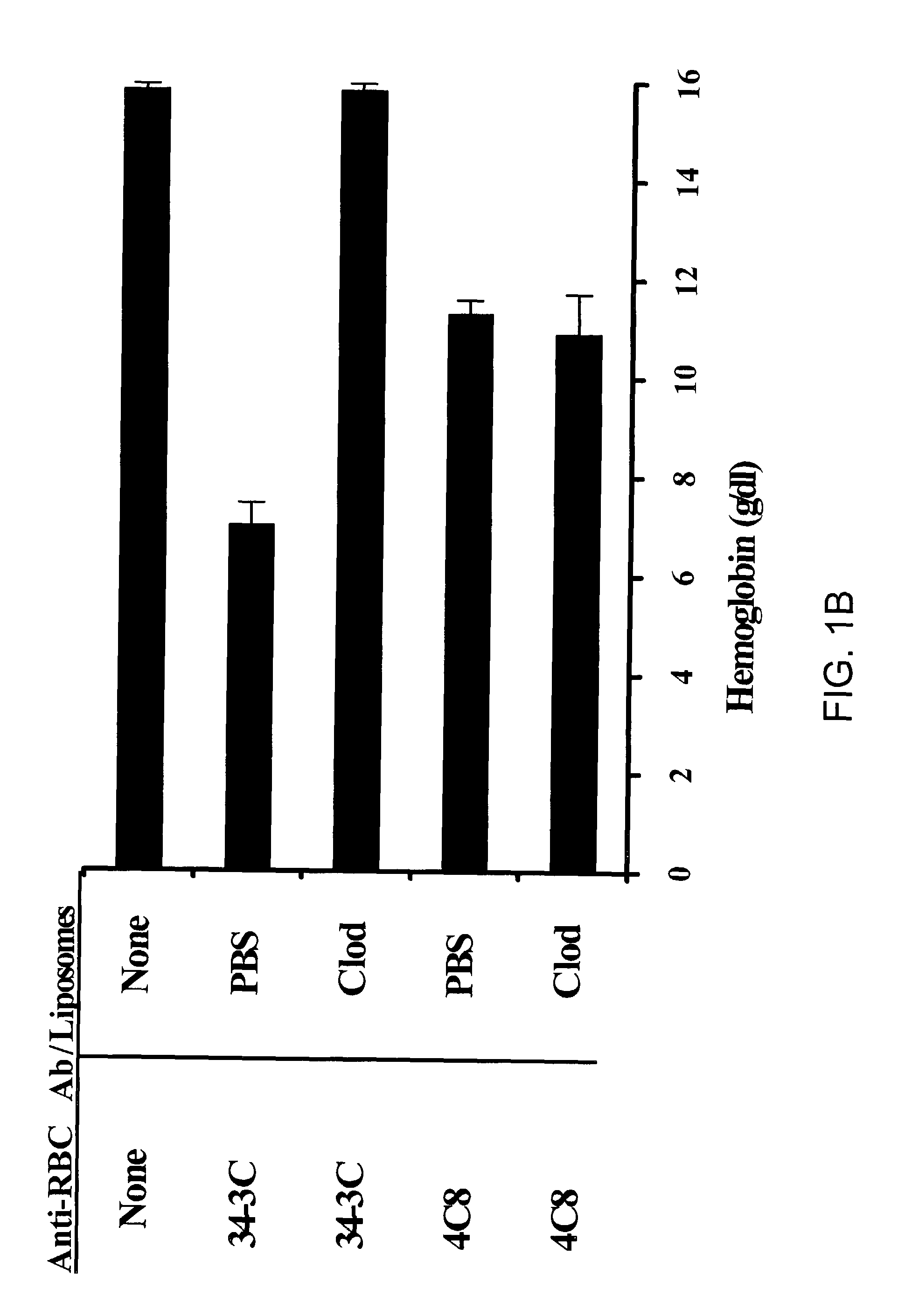
Composition and method for treating autoimmune hemolytic anemia
ActiveUS7090865B2Reduce in quantityReduce gapBiocidePhosphorous compound active ingredientsAutoimmune hemolytic anemiaLiposome
Disclosed are a composition and method to treat or prevent antibody-induced anemia and particularly, autoimmune hemolytic anemia. The composition comprises a bisphosphonate and a pharmaceutically acceptable carrier. In a preferred embodiment, the composition comprises clodronate and a liposome carrier.
Owner:NAT JEWISH MEDICAL & RES CENT
Varicella zoster virus vaccine
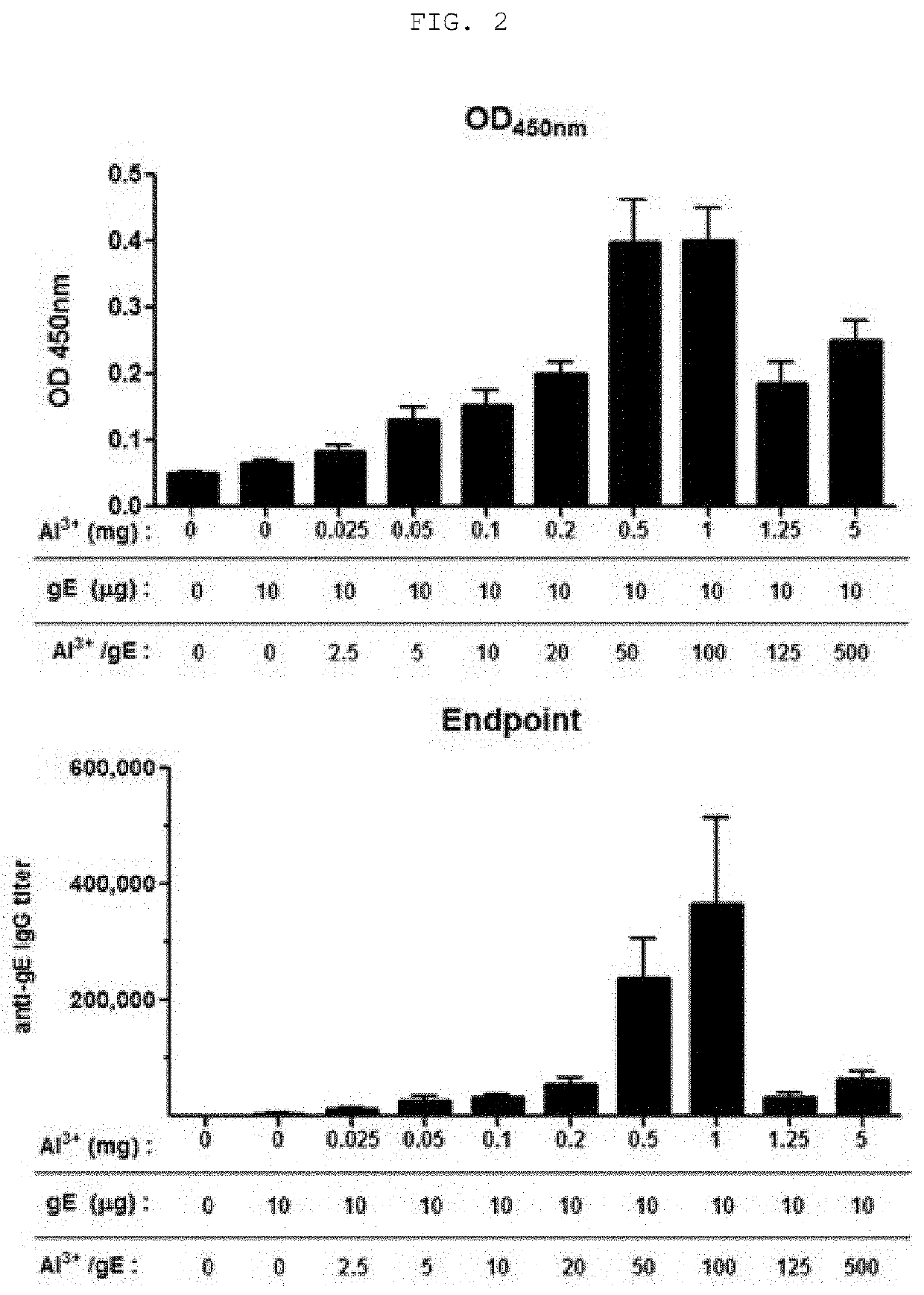
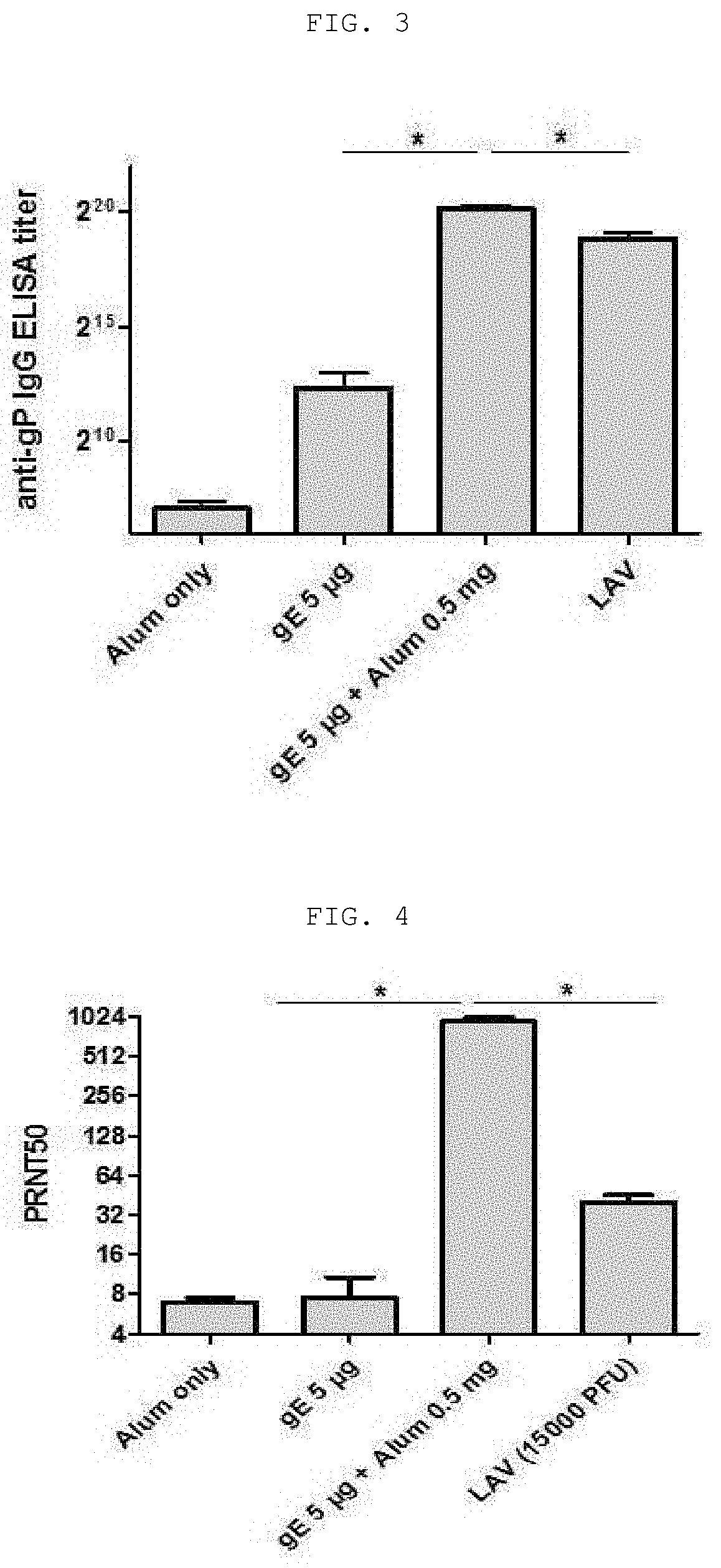
The present invention relates to a vaccine composition for prevention or treatment of chicken pox or herpes zoster, the vaccine composition comprising a surface protein (gE) of Varicella Zoster Virus and especially an aluminum salt as an adjuvant. The vaccine composition according to the present invention employs a protein antigen, thus showing greater outstanding stability than a live vaccine and has an optimized mixture ratio of adjuvants to elicit effective antibody induction, thereby being useful as a vaccine for preventing or treating Varicella Zoster Virus-caused chicken pox or herpes zoster.
Owner:MOGAM INST FOR BIOMEDICAL RES
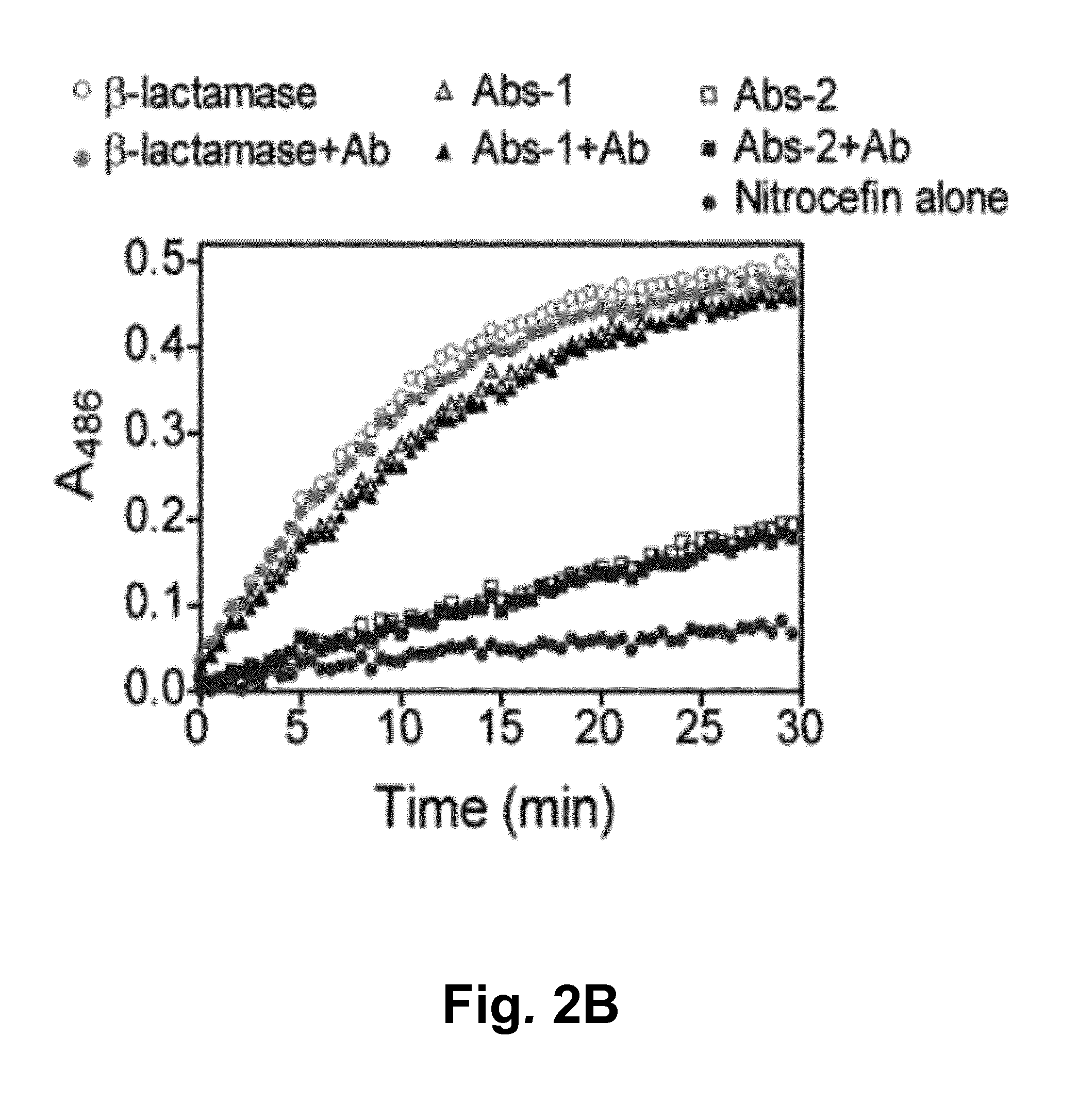
Switchable Reporter Enzymes for Homogenous Antibody Detection
InactiveUS20150285818A1Increase enzyme activityAchieve modularityBiological testingScreening proceduresAntibody Classes
A generic biosensor strategy was developed for the construction of switchable antibody reporter enzymes that allow direct detection of antibodies in solution including serum. The biosensor principle is based on the antibody-induced disruption of the intramolecular interaction between a reporter enzyme and its inhibitor and takes advantage of a unique structural property shared by all antibody classes, the presence of two identical antigen binding sites separated by a distance of approximately 100 Å. Unlike previous strategies, this biosensor design is intrinsically modular, allowing the construction of e.g. β-lactamase reporter enzymes for in principle any target antibody without cumbersome optimization / screening procedures. General guidelines are provided for the construction of reporter enzymes using enzyme-inhibitor pairs.
Owner:TECH UNIV EINDHOVEN
N-Linked Glycosylation Alteration in E0 and E2 Glycoprotein of Classical Swine Fever Virus and Novel Classical Swine Fever Virus Vaccine
InactiveUS20110038886A1Reduce severityAltered glycosylation patternSsRNA viruses positive-senseVirus peptidesClassical swine fever virus CSFVTGE VACCINE
E2 is one of the three envelope glycoproteins of Classical Swine Fever Virus (CSFV). E2 is involved in several functions including virus attachment and entry to target cells, production of antibodies, induction of protective immune response in swine, and virulence. Seven putative glycosylation sites in E2 were modified by site directed mutagenesis of a CSFV Brescia infectious clone (BICv). A panel of virus mutants was obtained and used to investigate whether the removal of putative glycosylation sites in the E2 glycoprotein would affect viral virulence / pathogenesis in swine. We observed that rescue of viable virus was completely impaired by removal of all putative glycosylation sites in E2, but restored when mutation N185A reverted to wild-type asparagine produced viable virus that was attenuated in swine. Single mutations of each of the E2 glycosylation sites showed that amino acid N116 (N1v virus) was responsible for BICv attenuation. N1v efficiently protected swine from challenge with virulent BICv at 3 and 28 days post-infection suggesting that glycosylation of E2 could be modified for development of CSF live-attenuated vaccines. Additionally, a new developed virus, contained deletions of putative glycosylation sites N1 in E2 and N1 in E0 (6b), called N1E0 / 2v, induce a solid protection against the challenge at 3 and 28 days post-inoculation.
Owner:UNITED STATES OF AMERICA +1
Biological Therapeutics for Infection-Relating Disorders or Conditions
ActiveUS20160024186A1Protection from damageSymptoms improvedViral antigen ingredientsImmunoglobulins against virusesTherapeutic antibodySerum ige
The present invention discloses products and the methods of uses of the products for preventing and treating infectious diseases and the disorders or conditions inducible by harmful antibodies. The harmful antibodies are induced during infection, or vaccination, or use of therapeutic antibodies. The products of the present disclosure comprise immunoglobulin products, serum or plasma, specific antibodies to viral pathogens.
Owner:B&H BIOTECH LLC
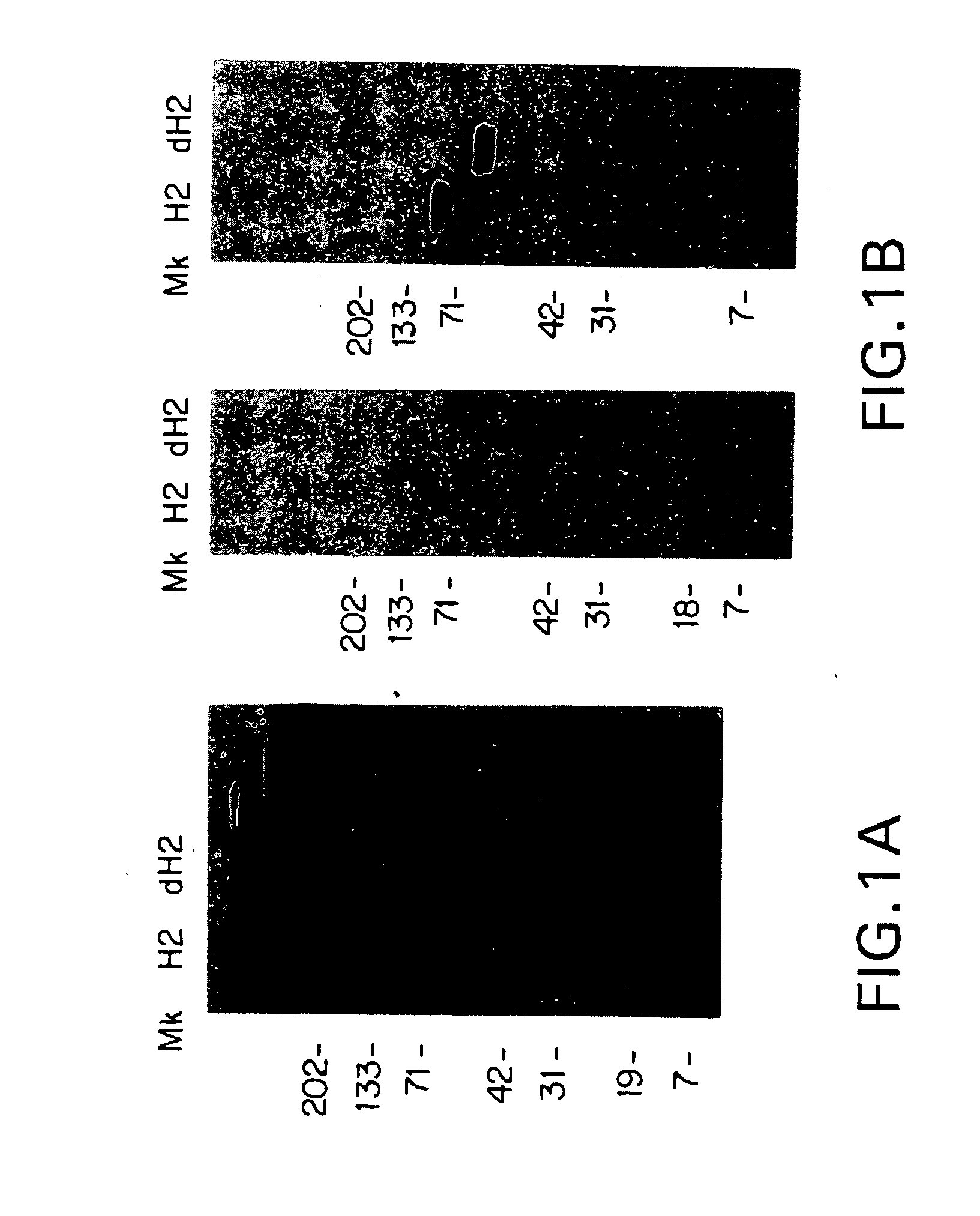
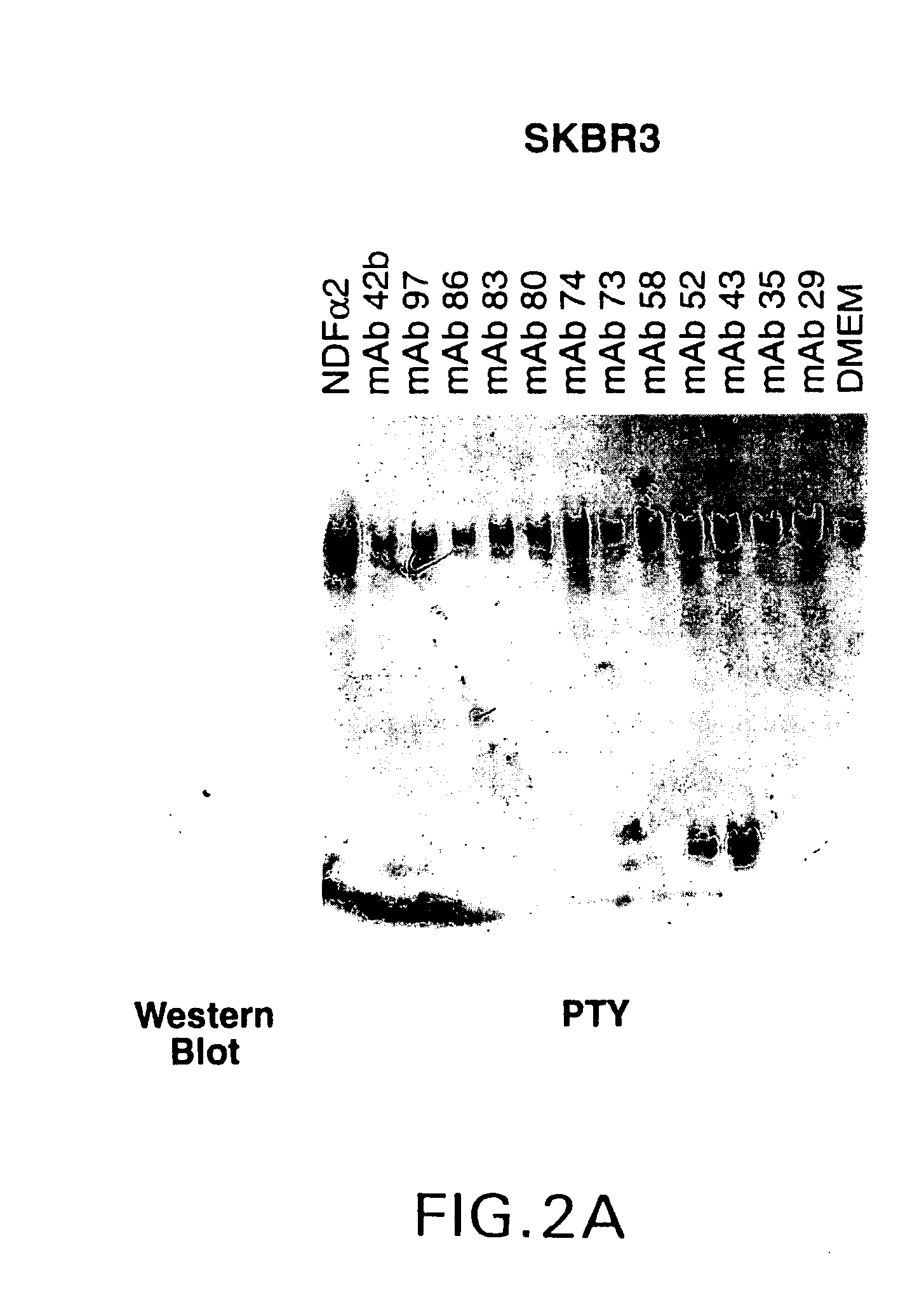
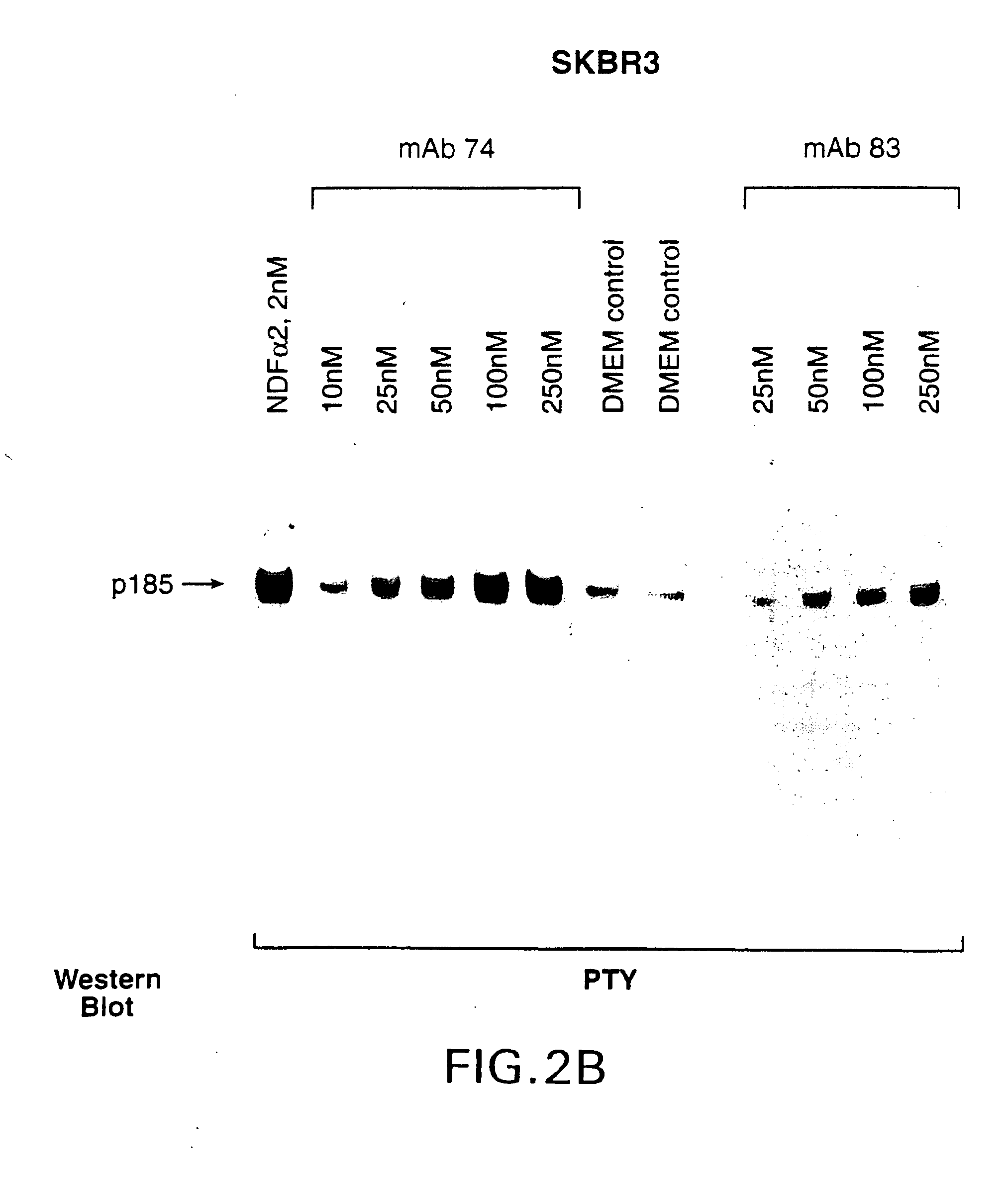
Antibody-induced apoptosis
Anti-Her2 antibodies which induce apoptosis in Her2 expressing cells are disclosed. The antibodies are used to “tag” Her2 overexpressing tumors for elimination by the host immune system. Also disclosed are hybridoma cell lines producing the antibodies, methods for treating cancer using the antibodies, and pharmaceutical compositions.
Owner:AMGEN INC
Recombinant Nucleic Acid of Seneca Valley Virus, Recombinant Vaccine Strain and Preparation Method and Use Thereof
PendingUS20220031832A1Large capacityStrong antibody induction activitySsRNA viruses positive-senseViral antigen ingredientsAntigenHighly pathogenic
The disclosure provides a recombinant nucleic acid of Seneca valley virus, a recombinant vaccine strain and preparation method and use thereof, and relates to the technical field of genetic engineering. The disclosure provides the recombinant nucleic acid of Seneca valley virus, recombinant Seneca valley virus comprising the recombinant nucleic acid, recombinant Seneca valley virus encoded by the recombinant nucleic acid, recombinant Seneca valley virus vaccine strain comprising the recombinant Seneca valley virus and preparation method and use thereof. According to the disclosure, a vaccine strain characterized by high antigen production capacity, remarkably reduced pathogenicity (even having no pathogenicity to pigs), strong antibody induction activity, high immune protection rate is prepared. The vaccine strain remarkably improves the biological safety and can be used for preventing and controlling Seneca valley virus in China and the neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Immunizing peptide, method for producing immunizing peptide, pharmaceutical composition for immune disease containing same, and method for treating immune disease
InactiveUS20180064792A1Induce productionImmunoglobulin superfamilyPeptide/protein ingredientsProtein targetProtein C
The present invention provides a novel immunizing peptide that can induce production of an antibody to a complex of the immunizing peptide and an MHC molecule of a living organism The immunizing peptide according to the present invention includes a T-cell epitope and an antibody-inducing portion of a target protein. The antibody-inducing portion is at least one of the following amino acid residues in an amino acid sequence of the target protein: one or more contiguous amino acid residues immediately upstream of a N-terminal amino acid residue of the T-cell epitope; and one or more contiguous amino acid residues immediately downstream of a C-terminal amino acid residue of the T-cell epitope. When the immunizing peptide is administered to a living organism, the immunizing peptide induces production of an antibody to a complex of the immunizing peptide and an MHC molecule of the living organism.
Owner:OSAKA UNIV
ANTIBODY BINDING SPECIFICALLY TO CD66c AND USE THEREOF
ActiveUS20190263905A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen Binding Fragment
The present invention relates to an anti-CD66c antibody and its use for treating cancer, and more particularly, it is possible to induce T-cell activation or humoral immune response using an antibody specifically recognizing CD66c. A nucleic acid molecule encoding the antibody or antigen-binding fragment thereof, a vector comprising the nucleic acid molecule, a host cell and the antibody or antigen-binding fragment thereof is used for alleviation, prevention, treatment or diagnosis of CD66c-related disease.
Owner:KUMHO HT
Biological Therapeutics for Infection-Relating Disorders or Conditions
ActiveUS20190270792A1Protection from damageSymptoms improvedViral antigen ingredientsMicrobiological testing/measurementVaccinationBlood plasma
The present invention discloses products and the methods of uses of the products for preventing and treating infectious diseases and the disorders or conditions inducible by harmful antibodies. The harmful antibodies are induced during infection, or vaccination, or use of therapeutic antibodies. The products of the present disclosure comprise immunoglobulin products, serum or plasma, specific antibodies to viral pathogens.
Owner:B&H BIOTECH LLC
Treatment of multiple sclerosis by alemtuzumab induction followed by laquinimod therapy
This invention provides a method of treating a subject afflicted with multiple sclerosis (MS) or presenting clinically isolated syndrome (CIS) which comprises a) administering to the subject an amount of an anti-CD52 antibody, followed by b) periodically administering to the subject an amount of laquinimod. This invention also provides packages comprising pharmaceutical compositions of laquinimod or an anti-CD52 antibody for treating such a subject wherein laquinimod is to be administered as a maintenance therapy in such a subject who has received an anti-CD52 antibody induction therapy.
Owner:TEVA PHARMA IND LTD
Immunizing peptide, method for producing immunizing peptide, pharmaceutical composition for immune disorders containing same, and method for treating immune disorders
InactiveCN107735408AImmunoglobulin superfamilyPeptide/protein ingredientsCellular antigensProtein target
Provided is a novel immunizing peptide with which it is possible to induce antibody production to complexes that include the immunizing peptide and the MHC molecule of a living body. The immunizing peptide of the present invention is characterized in that: the immunizing peptide includes a T-cell epitope and antibody induction portion of a target protein; the antibody induction portion comprises one or more consecutive amino acids sequences upstream from the N-end amino acid of the T-cell epitope, and / or one or more consecutive amino acids sequences downstream from the C-end amino acid of theT-cell epitope, in the amino acid sequence of the target protein; and, when administered to a living body, the immunizing peptide induces antibody production to complexes that include the immunizing peptide and the MHC molecule of the living body.
Owner:OSAKA UNIV
Biological therapeutics for infection-relating disorders or conditions
ActiveUS10202439B2Protection from damageSymptoms improvedViral antigen ingredientsImmunoglobulins against virusesSerum igeTherapeutic antibody
The present invention discloses products and the methods of uses of the products for preventing and treating infectious diseases and the disorders or conditions inducible by harmful antibodies. The harmful antibodies are induced during infection, or vaccination, or use of therapeutic antibodies. The products of the present disclosure comprise immunoglobulin products, serum or plasma, specific antibodies to viral pathogens.
Owner:B&H BIOTECH LLC
Inactivated whole-virus influenza vaccine and method for preparing same
PendingUS20220031833A1High antibody-inducing abilityReduced pyrogenicitySsRNA viruses negative-senseViral antigen ingredientsEmbryonated chicken eggVirus influenza
An inactivated whole-virus influenza vaccine may have its antibody-inducing ability is maintained or enhanced and may cause fewer side reactions. A method for preparing an inactivated whole-virus influenza vaccine may use an embryonated chicken egg method, including subjecting a virus solution including a whole influenza virus collected from embryonated chicken eggs to a hypotonic treatment.
Owner:DENKA CO LTD +1
Autoimmune disease model animal
InactiveUS20060168666A1Stable productionShow phenotype of autoimmune diseaseBiocideCell receptors/surface-antigens/surface-determinantsOral mucosa blistersImmunologic disorders
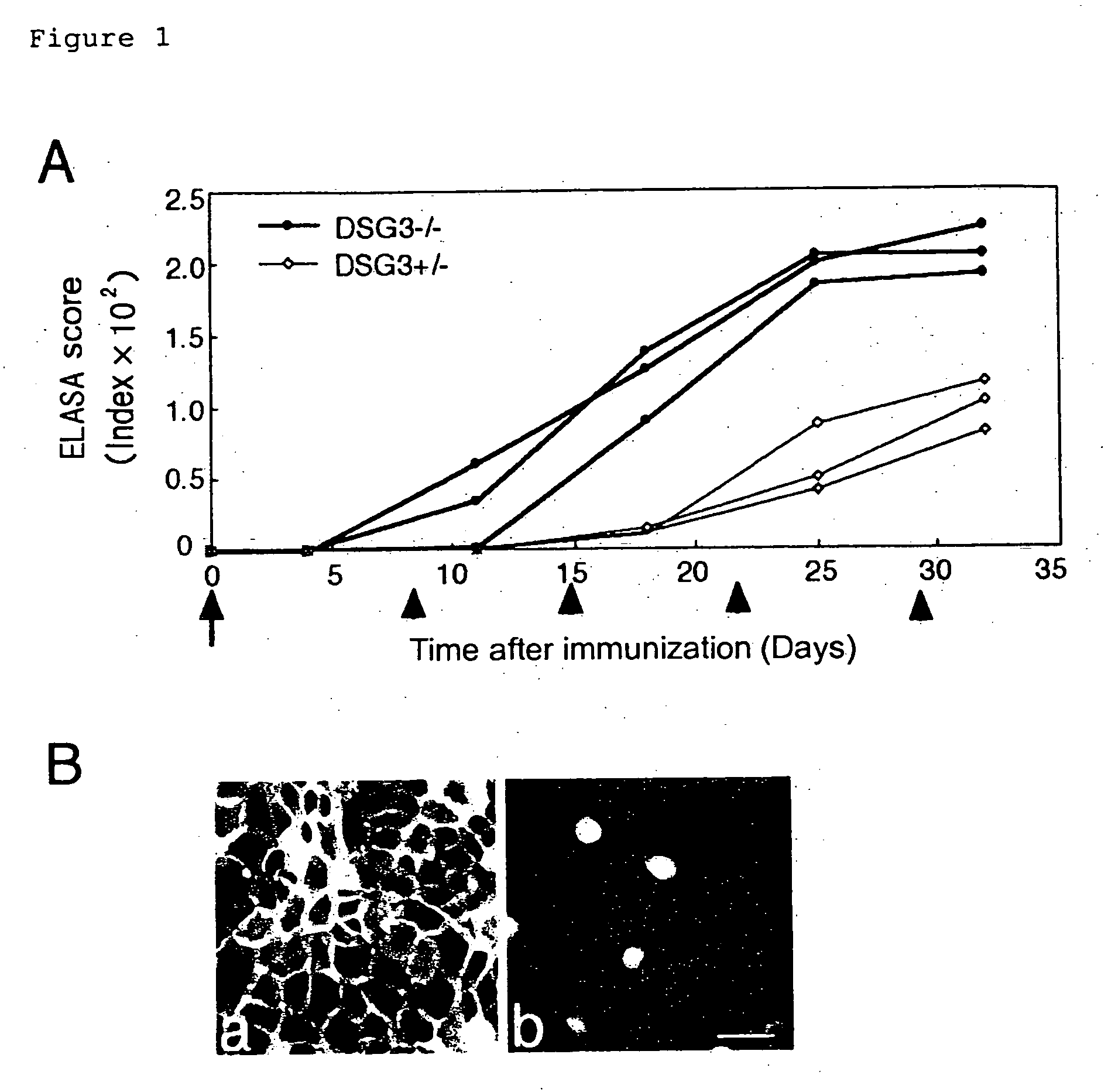
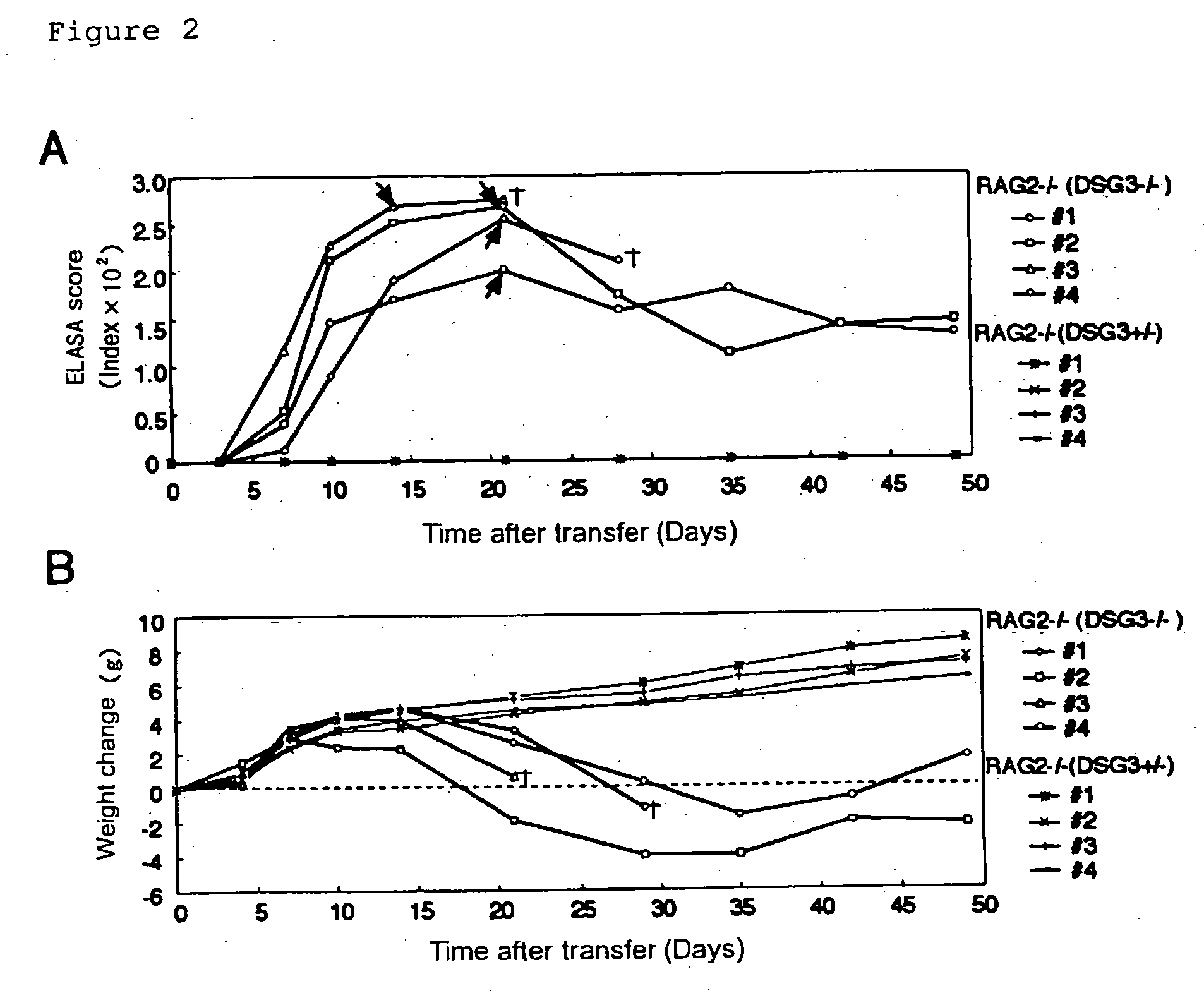
Pemphigus vulgaris (PV) is an autoimmune disease with a possible fatality of the skin and mucosae which is induced by an antibody against desmoglein 3 (Dsg3). Persistent production of anti-Dsg3 IgG can be induced by adoptively transferring spleen cells of a DSG3− / − mouse immunized with rDsg3 into an RAG2− / − immunodeficient mouse expressing Dsg3 protein. This IgG in the blood binds to the Dsg3 protein in vivo, induces the breakage of intercellular adhesion of keratinocytes and thus brings about the phenotype of pemphigus vulgaris involving the formation of blisters in the oral mucosa and the disappearance of resting hair. These effects are sustained over 6 months. By using this method, active disease model animals relating to various autoimmune diseases can be constructed.
Owner:KEIO UNIV
Treatment of multiple sclerosis by alemtuzumab induction followed by laquinimod therapy
InactiveUS20160296513A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMaintenance therapyAntiendomysial antibodies
This invention provides a method of treating a subject afflicted with multiple sclerosis (MS) or presenting clinically isolated syndrome (CIS) which comprises a) administering to the subject an amount of an anti-CD52 antibody, followed by b) periodically administering to the subject an amount of laquinimod. This invention also provides packages comprising pharmaceutical compositions of laquinimod or an anti-CD52 antibody for treating such a subject wherein laquinimod is to be administered as a maintenance therapy in such a subject who has received an anti-CD52 antibody induction therapy.
Owner:TEVA PHARMA IND LTD
Preparation of flagellin vaccine adjuvant-based vaccine to induce production of antibody recognizing conformation of antigens, and application thereof
PendingCN110312524AStrong specificityEfficient use ofBacterial antigen ingredientsNervous disorderNeuro-degenerative diseaseViral Vaccine
The present invention provides a vaccine composition for use in neurodegenerative diseases and an infectious virus vaccine composition for inducing an antibody recognizing the conformation of antigens. The vaccine composition of the present invention induces the production of an antibody recognizing the conformation of antigens. The antibody recognizing the conformation of antigens has high specificity for an antigen, and thus can be useful for ameliorating, preventing or treating diseases.
Owner:李浚行 +4
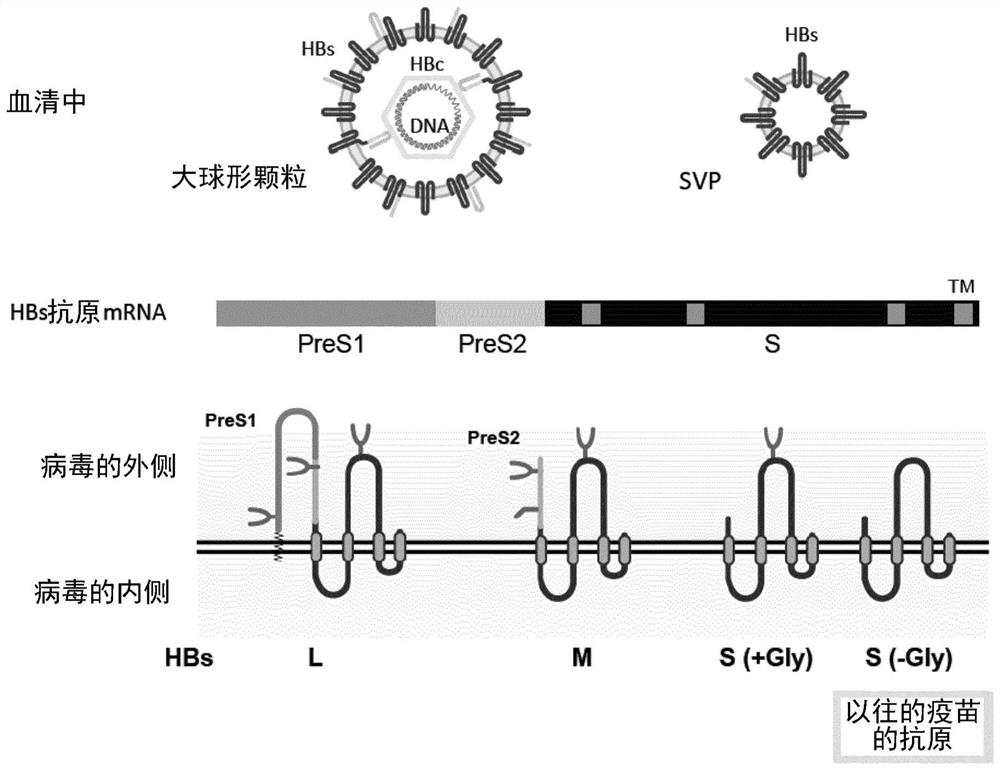
Method for efficiently inducing antibody to hepatitis virus, antibody and detection system
To provide an examination system whereby an antigen having a sugar chain added thereto can be recognized in Dane particle of hepatitis B virus (HBV), and a neutralizing antibody that recognizes an antigen having a sugar chain added thereto and exhibits an inhibitory effect on infection. By clarifying that Dane particle relates to a specific sugar chain structure, it becomes possible to construct anovel detection system for hepatitis B virus particles which show infectivity, i.e., contain a nucleic acid. Further, it becomes possible thereby to provide a neutralizing antibody that recognizes anantigen having a sugar chain added thereto and exhibits an inhibitory effect on infection.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Antibody binding specifically to CD66c and use thereof
ActiveUS11220543B2Efficient detectionMaximize the effectBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen Binding Fragment
The present invention relates to an anti-CD66c antibody and its use for treating cancer, and more particularly, it is possible to induce T-cell activation or humoral immune response using an antibody specifically recognizing CD66c. A nucleic acid molecule encoding the antibody or antigen-binding fragment thereof, a vector comprising the nucleic acid molecule, a host cell and the antibody or antigen-binding fragment thereof is used for alleviation, prevention, treatment or diagnosis of CD66c-related disease.
Owner:KUMHO HT
Peptide which can induce antibody capable of recognizing stereostructure of HIV
InactiveCN103502264AImprove featuresHigh activityViral antigen ingredientsVirus peptidesNeutralising antibodyMolecular binding
The purpose of the present invention is to provide a peptide which can induce an excellent or novel neutralizing antibody against an HIV, and thereby prevent or treat HIV infection or expand choices of the prevention or treatment. A means for achieving the purpose is characterized by using a peptide which can induce an antibody capable of recognizing the stereostructrue of an HIV, wherein the peptide can recognize a trimeric region in C34 and is characterized in that three molecules of a derivative of a helical region C34 peptide located on the C-terminal side of a transmembrane protein gp41 of an HIV particle are bound together through a template compound that has three equivalent linker structures in a C3 symmetric manner.
Owner:NAT UNIV CORP TOKYO MEDICAL & DENTAL UNIV
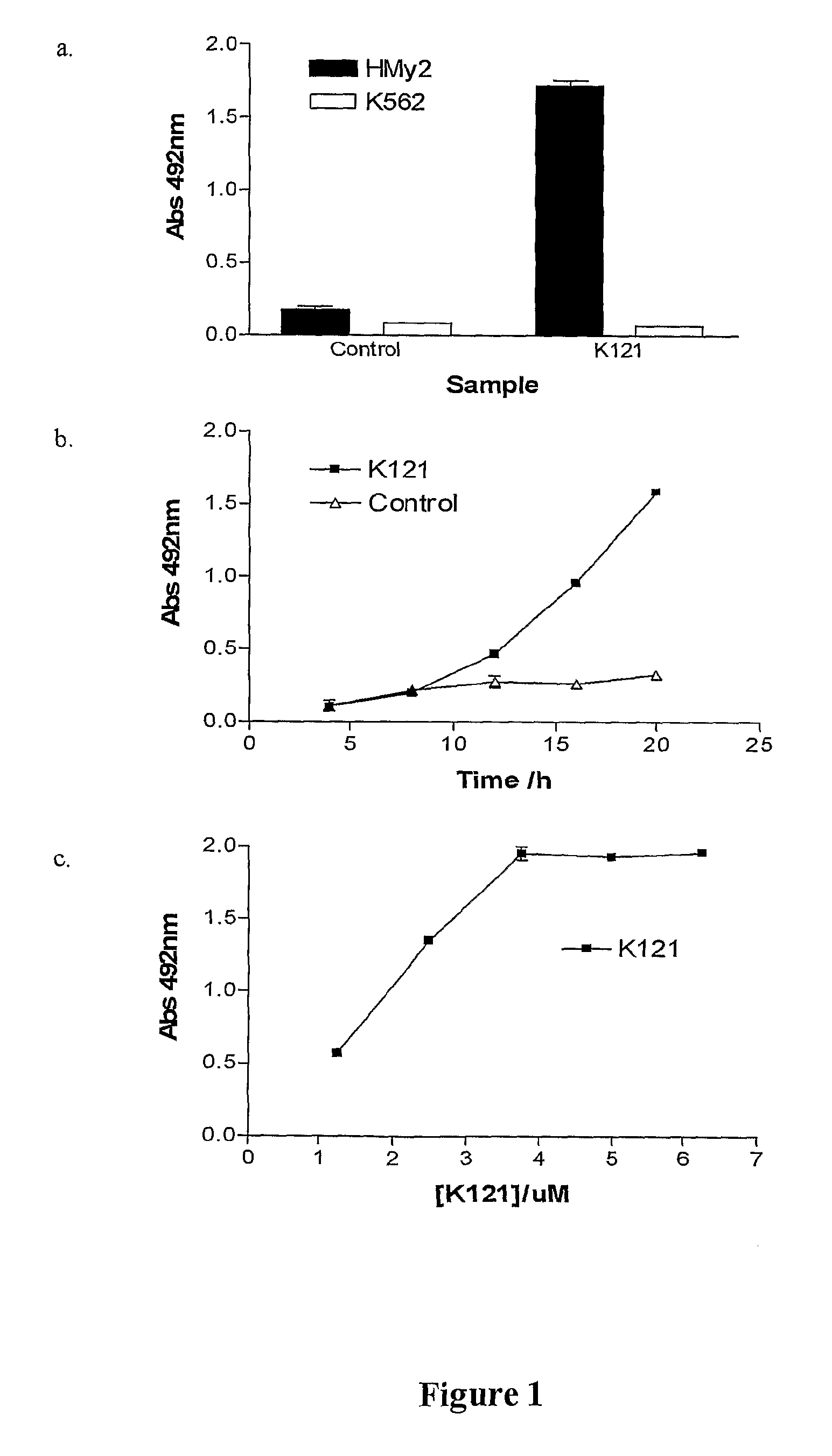
Method for treating multiple myeloma
InactiveUS20080199463A1Growth inhibitionAntibody mimetics/scaffoldsGenetic material ingredientsApoptosisMyeloma cell
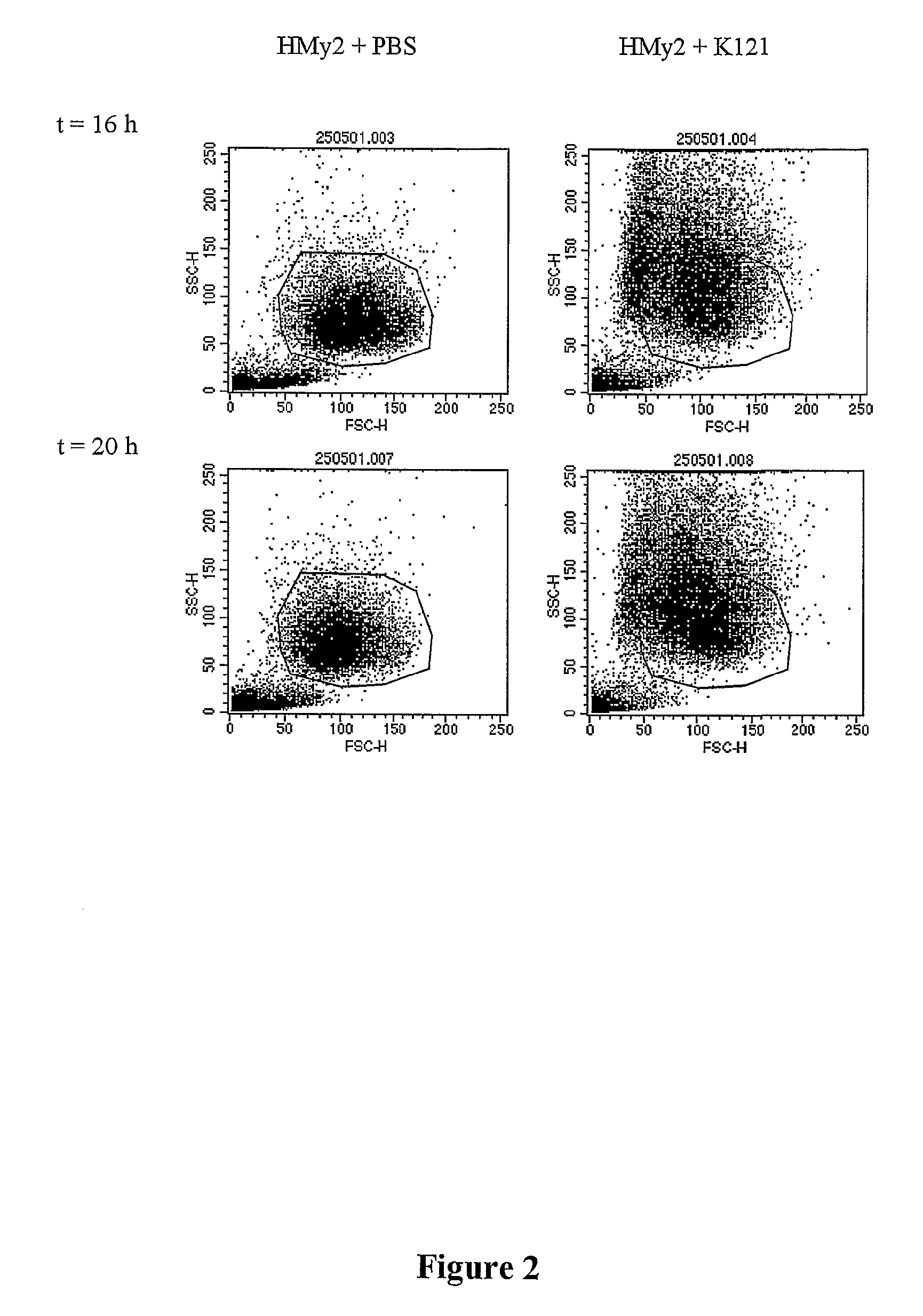
The present invention relates to methods for the treatment of multiple myeloma. More particularly, the present invention relates to a method for inducing apoptosis in myeloma cells by administration of a K121-like antibody.
Owner:HAEMALOGIX PTY LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com