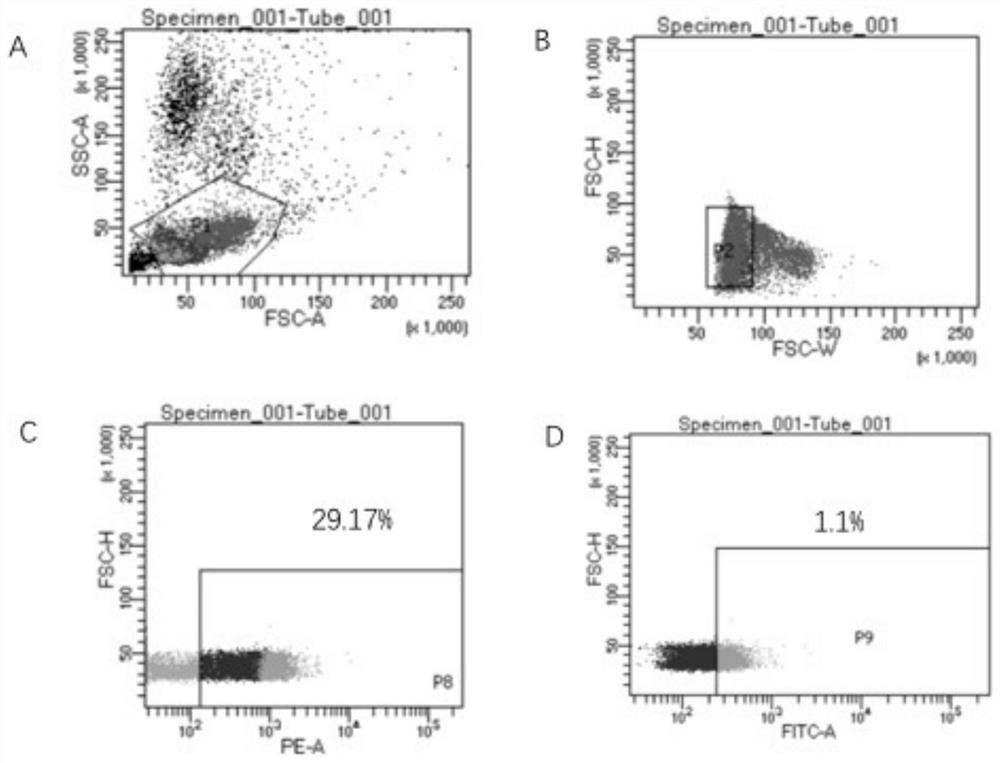
Screening method of single memory b cells and its application in the preparation of small molecule monoclonal antibodies
A monoclonal antibody, chloramphenicol technology, applied in the field of cell biology and immunology, can solve the problems of easy volatilization and degradation, insufficient reverse transcription, low-efficiency amplification, etc., to achieve high affinity, high practical value, The effect of overcoming technical barriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
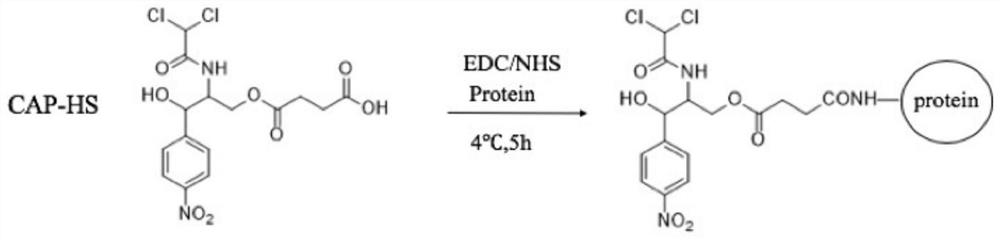
[0050]The preparation method of chloramphenicol immunogen CAP-KLH is as follows: 1 mg CAP-HS is dissolved in 500 μL DMF, 2.75 mg NHS is dissolved in 200 μL DMF, and 2.75 mg EDC is dissolved in 400 μL DMF. 500 μL of the reaction solution was slowly added dropwise to 2 mg of KLH, and the reaction was shaken at 4°C overnight. Another 500 μL of the reaction solution was added with 2 mg BSA, and dialyzed for 72 h. The solution obtained from coupling was aliquoted and stored at -20°C ( figure 1 ).
[0051] The immunological formulation was prepared as follows: CAP-KLH (at a concentration of 4 mg / mL) was mixed with complete Freund's adjuvant / incomplete Freund's adjuvant in equal volumes and emulsified.
[0052] The immunization method is as follows: multi-point injection immunization method on the neck and back. The number of immunizations was 8. The first immunization was the primary immunization with complete Freund's adjuvant, and the other immunizations were performed with inc...
Embodiment 1
[0064] Example 1 Immunization of New Zealand White Rabbit
[0065] The concentration of the immunogen (CAP-KLH) was adjusted to 4 mg / mL with PBS buffer, and the concentration of the immunogen was determined by the Bicinchoninic Acid method.
[0066] Take 6 New Zealand white rabbits (2 months old, weight 2.5kg), numbered CAP-KLH-1, CAP-KLH-2, CAP-KLH-3, CAP-KLH-4, CAP-KLH-5 and CAP -KLH-6. New Zealand white rabbits were immunized eight times, and the immunization method was 4-8 points on the back of the neck.
[0067] The first immunization: each animal was immunized with 1 mL of the first-immune preparation (each 1 mL of the first-immune preparation contained 1 mg of CAP-KLH).
[0068] The second to eighth immunizations: counting the days from the first immunization, one immunization every 30 days for a total of 7 booster immunizations; the first 3 boosters, each immunized with 1mg CAP-KLH; the last 3 boosters Immunization, 800μg, 600μg and 400μg, respectively. One week aft...
Embodiment 2
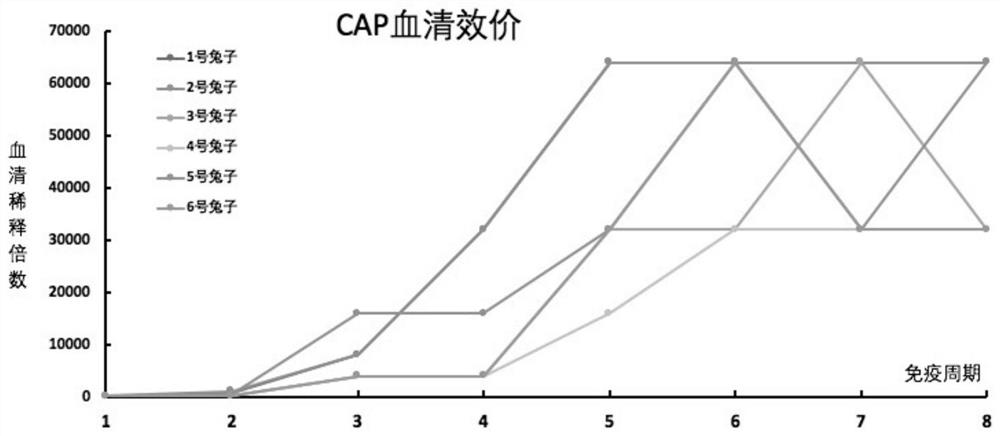
[0069] Example 2 Affinity identification of New Zealand white rabbit antiserum
[0070] Antisera obtained one week after immunization were tested for titer and sensitivity. The assay method is indirect ELISA. The specific operation steps are as follows:
[0071] The coated original CAP-BSA was diluted with carbonate buffer (pH 9.6) to 0.1 μg / mL, coated with 100 μL / well of ELISA plate, incubated at 37°C for 2 h, and then washed three times with PBST solution. The ELISA plate was then blocked with 2% nonfat dry milk (150 μL / well), incubated at 37° C. for 1 h, washed three times with PBST solution, and patted dry. The antiserum was serially diluted with PBS, starting from a 1:4000 dilution, with a 2-fold gradient, and a total of 8 dilutions. Add 50 μL of PBS buffer and 50 μL of serially diluted antiserum solution to the coated ELISA plate, incubate at 37°C for 30 min, then wash with PBST solution for 3 times and pat dry. 100 μL of enzyme-labeled secondary antibody diluent (ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com