Treatment of diseases by liver expression of an enzyme which has a deoxyribonuclease (DNASE) activity
A technology of DNase and nucleic acid, which is applied in the field of treating diseases by expressing enzymes with deoxyribonuclease (DNase) activity through the liver, which can solve limited problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0376] Example 1: Limited Ability of DNase to Digest Circulating Cell-Free DNA (cfDNA) in Cancer Patients
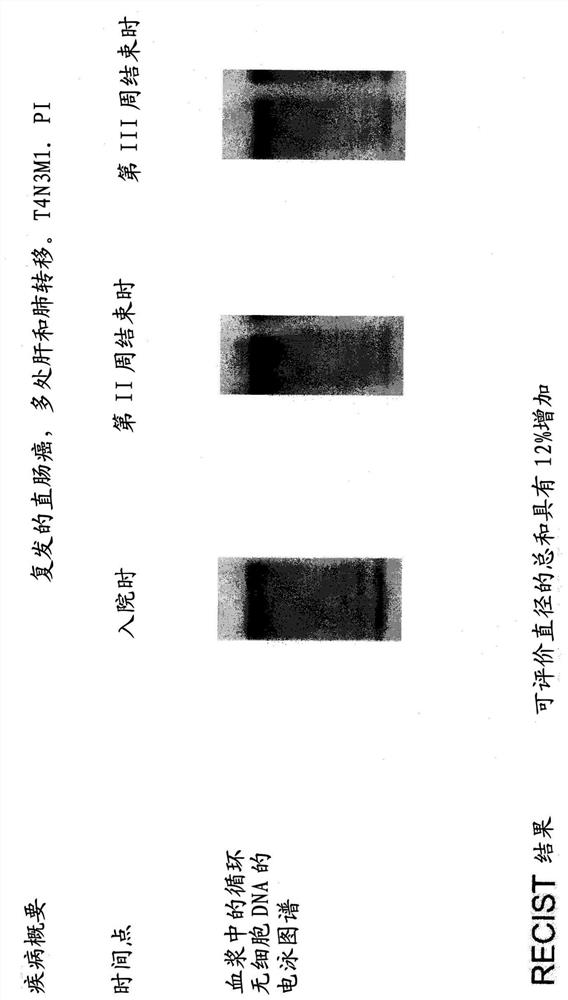
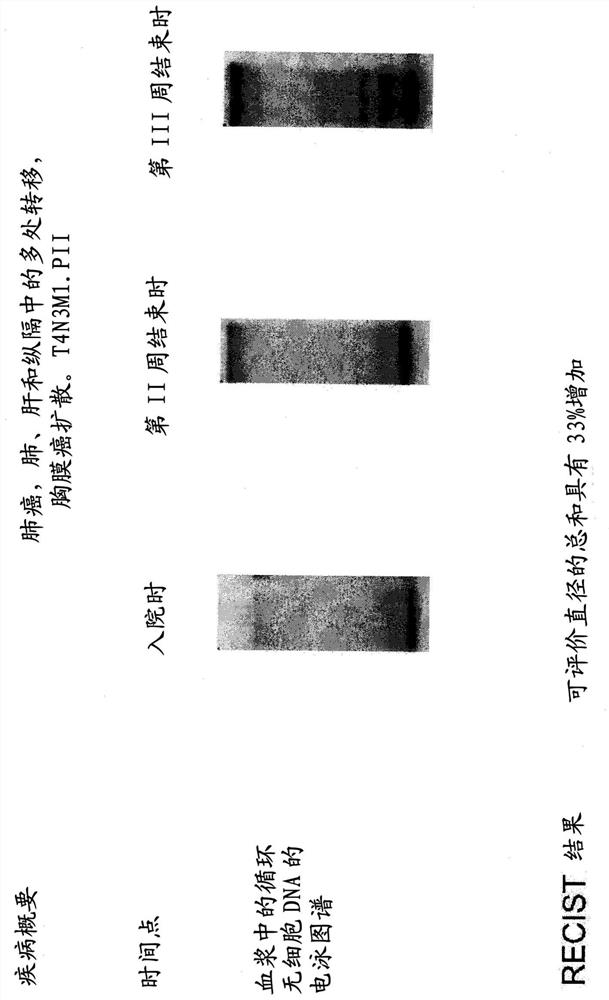
[0377] Nine patients with advanced metastatic disease recruited at the Department of Thoracic Surgery of Kostushko Hospital (St. Russian Samson). Treatment was carried out according to the following schedule: 300 mg per day by six 30-minute intravenous infusions during week I; 450 mg per day by six 30-minute intravenous infusions during week II; and 600 mg per day by six 30-minute intravenous infusions.
[0378] Electrophoretic profiling of circulating cfDNA was performed in each patient before the first infusion of DNase I protein, at the end of week II, and immediately after the last infusion of DNase I protein. DNA was isolated from blood plasma using the classical phenol-chloroform method. Blood cfDNA electrophoresis was performed on a 1% agarose gel. DNA was visualized with ethidium bromide. One week after the last infusion of DNase I protein, treatment effic...
Embodiment 2
[0381] Example 2: Accumulation of cell-free DNA in the liver of tumor-bearing animals
[0382] will be approximately 12x 10 6 Two LS174T human colon cancer cells were inoculated subcutaneously into the left flank of twelve 8-week-old female nu / nu mice (IBCH RAS strain). Mice received one intravenous injection of 99mTc-labeled anti-DNA antibody according to the following schedule: three mice received the injection on D5, three mice received the injection on D10, three mice received the injection on D15, and three mice received the injection on D15. Injections were received on D25. Mice were sacrificed two hours after injection, and tissues and blood were collected for gamma counts.
[0383] AC-30-10 DNA-binding antibody (Genetex) was labeled with 99mTc as follows: a 0.024 mg / ml solution of the antibody in 0.3M citrate buffer (pH 4.2) was mixed with 1 mCi of 99mTc-high in 0.25 ml saline. Technetate was mixed and mixed with a 10-mg / ml solution of SnCl2 dihydrate in 0.1N HCl....
Embodiment 3
[0388] Example 3: Source supply of tumor-derived cfDNA from liver to blood
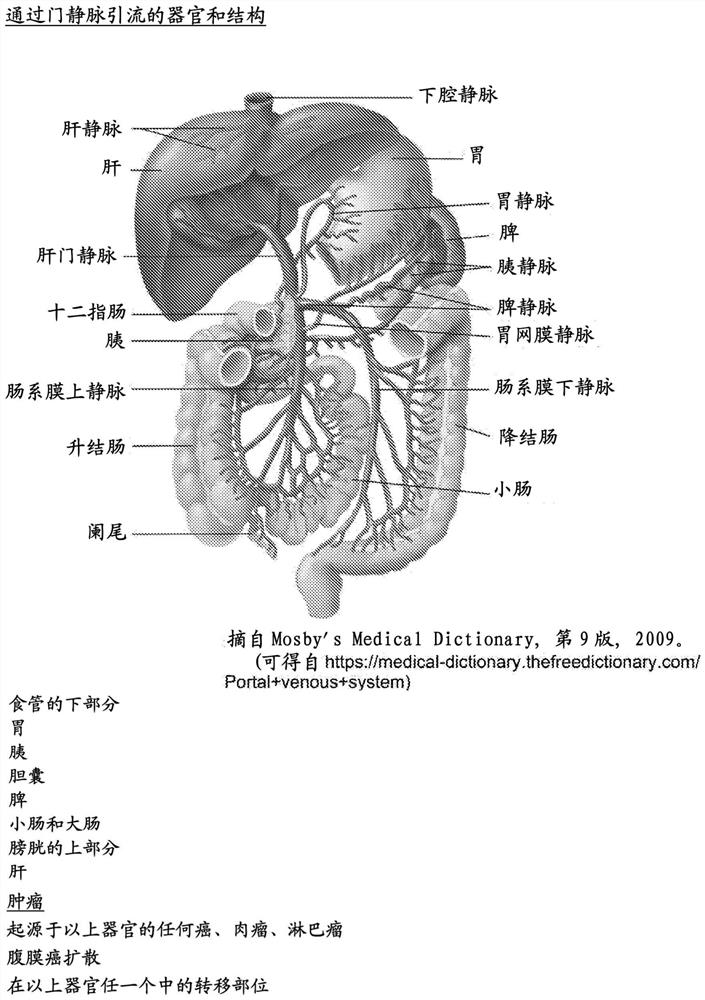
[0389] Six 6-week-old female nude mice (IBCH RAS species) were subcutaneously injected with 2x10 6 MCF-7 cells were injected subcutaneously into the left flank. On day 20 after injection, tumors were surgically removed. On day 25, all mice were anesthetized, and 500 μl blood samples were collected from the portal vein, hepatic artery and hepatic vein of each mouse. Plasma was separated from blood cells by centrifugation at 2000g for 10 minutes; the clear plasma phase on top was transferred to a new tube and centrifuged at 14000g. Transfer the supernatant to a new tube for DNA extraction. cfDNA was extracted from blood plasma samples using the QIAamp DNA Blood Mini Kit according to the manufacturer's instructions. The human β-globin sequence in the cell-free DNA was quantified in each sample by RT-PCR (iCycleriQ5Bio-Rad) using the human β-globin primer CAACTTCATCCACGTTCACC (SEQ ID NO: 10). Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com