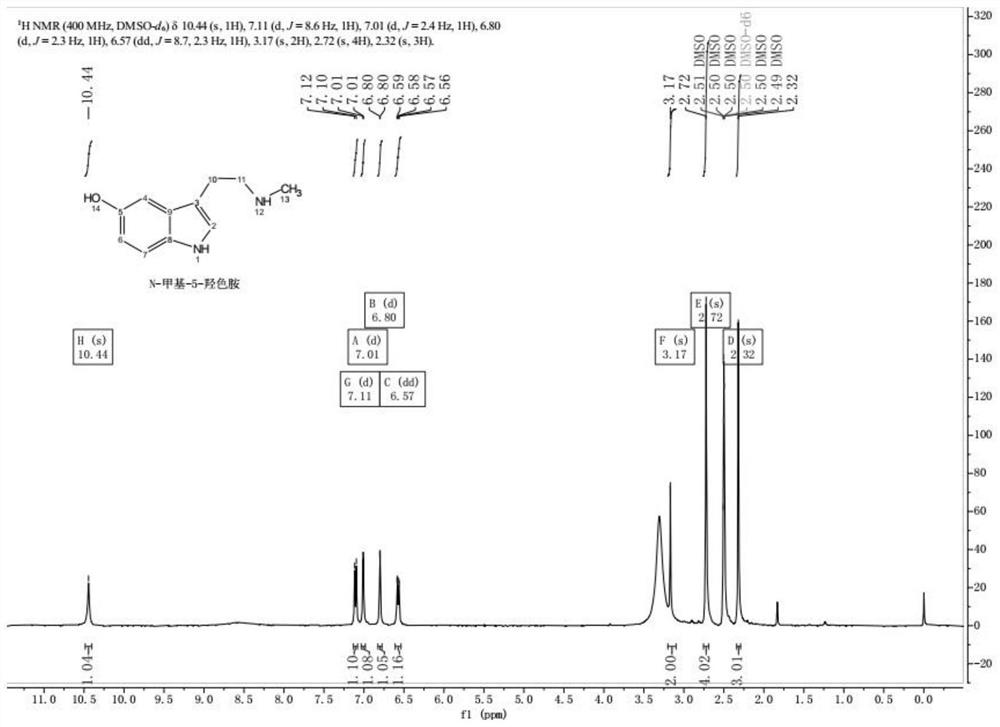
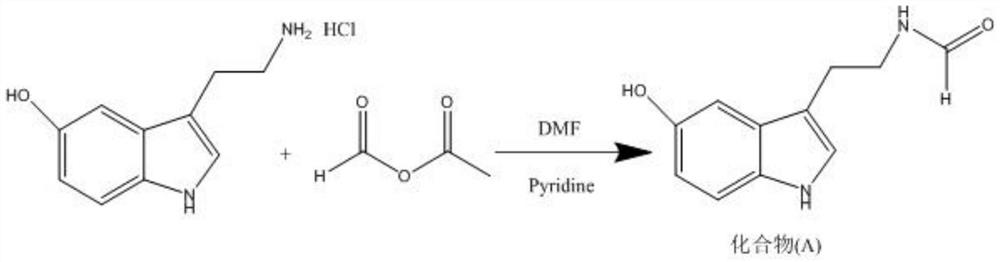
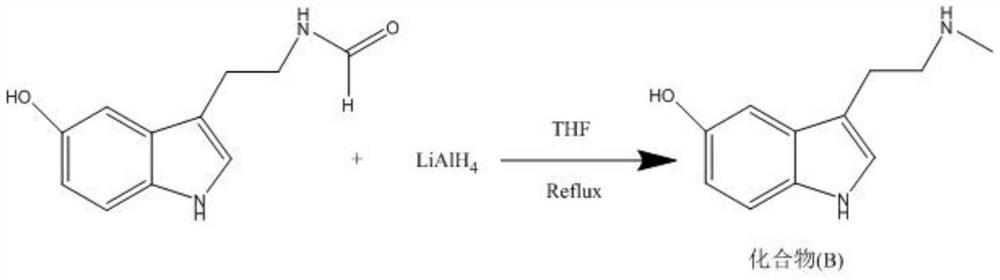
Method for preparing N-methyl-5-hydroxytryptamine by taking 5-hydroxytryptamine hydrochloride as raw material
A technology of serotonin hydrochloride and serotonin, which is applied in organic chemistry and other fields, and can solve the problems of complex components and low content of active ingredients of N-methyl-5-serotonin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 716ml of DMF solvent into a 2L three-necked flask, add 21.26g (0.100mol) of 5-hydroxytryptamine hydrochloride under stirring, and then add 20.14ml (0.250mol) of pyridine into the three-necked flask, keep the temperature at 25°C, and stir for 20min. Then put the three-necked flask into an ice-water bath, cool the reaction solution to 10°C, and then add 9.7g of formic acetic anhydride solution (0.110mol) dropwise to the three-necked flask, the dropping process controls the reaction temperature within 12-15°C, The time is 20 minutes. After the dropwise addition, keep at 12-15°C to continue the reaction for 2h.
[0032] Then add 477mL of deionized water under stirring, then add 286ml of dichloromethane, stir for 10min and then let stand for 5min, separate the organic phase of the lower layer of dichloromethane, then add 286ml of dichloromethane, stir for 10min and let stand for 5min, separate For the organic phase of dichloromethane in the lower layer, repeat this extr...
Embodiment 2
[0037] Add 830ml of DMF solvent into a 2L three-necked flask, add 21.26g (0.100mol) of 5-hydroxytryptamine hydrochloride under stirring, and then add 28.19ml (0.350mol) of pyridine into the three-necked flask, keep the temperature at 20°C, and stir for 10min. Then put the three-necked flask into an ice-water bath, cool the reaction solution to 10°C, and then add 11.44g of formic acetic anhydride solution (0.130mol) dropwise to the three-necked flask, and control the reaction temperature within 10-13°C during the dropping process. The time is 30 minutes. After the dropwise addition, keep at 10-13°C to continue the reaction for 1.5h.
[0038] Then add 550mL of deionized water under stirring, then add 330ml of dichloromethane, stir for 10min and let it stand for 5min, separate the lower layer of dichloromethane organic phase, then add 330ml of dichloromethane, stir for 10min and let stand for 5min, separate the lower layer of dichloromethane Chloromethane organic phase, repeat t...
Embodiment 3
[0043] Add 780ml of DMF solvent into a 2L three-necked flask, add 21.26g (0.100mol) of 5-hydroxytryptamine hydrochloride under stirring, and then add 24.16ml (0.300mol) of pyridine into the three-necked flask, keep the temperature at 20°C, and stir for 10min. Then put the three-necked flask into an ice-water bath, cool the reaction solution to 10°C, and then add 10.56g of formic acetic anhydride solution (0.120mol) dropwise to the three-necked flask, and control the reaction temperature within 12-14°C during the dropping process. The time is 30 minutes. After the dropwise addition, keep at 12-14°C to continue the reaction for 2h.
[0044] Then add 520ml of deionized water under stirring, then add 312ml of dichloromethane, stir for 10min and then let it stand for 5min, separate the organic phase of the lower layer of dichloromethane, then add 312ml of dichloromethane, stir for 10min and then let it stand for 5min, separate For the organic phase of dichloromethane in the lower ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com