Novel benzothiapyran diketone compound as well as preparation method and application thereof
A technology of benzothiopyrandione and thiopyrandione, which is applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of artificial synthesis and achieve good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of 8-methylbenzothiopyrandione, concrete steps are as follows:
[0028] (1) 2-Methyl-4H-benzothiopyran-4-one
[0029] 12.5g H 3 PO 4 , 18gP 2 O 5 Stir at 70°C, and after dissolving, add a mixture of 1.5 mL of thiophenol and 1.5 mL of ethyl acetoacetate dropwise (15 min) using a constant pressure dropping funnel. After reacting for 3 hours, it was poured into ice water and extracted with ethyl acetate. The organic layer was washed with 10% NaOH aqueous solution (20 mL×3), and adjusted to pH=9. After washing with water, the organic layer was washed with anhydrous MgSO 4 dry. The obtained product was concentrated and crystallized with diethyl ether to obtain 1.6 g of yellow crystals, with a yield of 77.4%.
[0030] 1 H-NMR (CDCl 3 / TMS) δ: 8.48(1H, J=7.9Hz, d), 7.56-7.52(2H, m), 7.50(1H, J=7.6, 7.9Hz, dd), 6.82(1H, s), 2.44(3H ,s).
[0031] 13 C-NMR (CDCl 3 )δ: 180.63, 151.32, 137.64, 131.35, 130.70, 128.54, 127.48, 125.98, 124.89, 23.31.
[00...
Embodiment 2
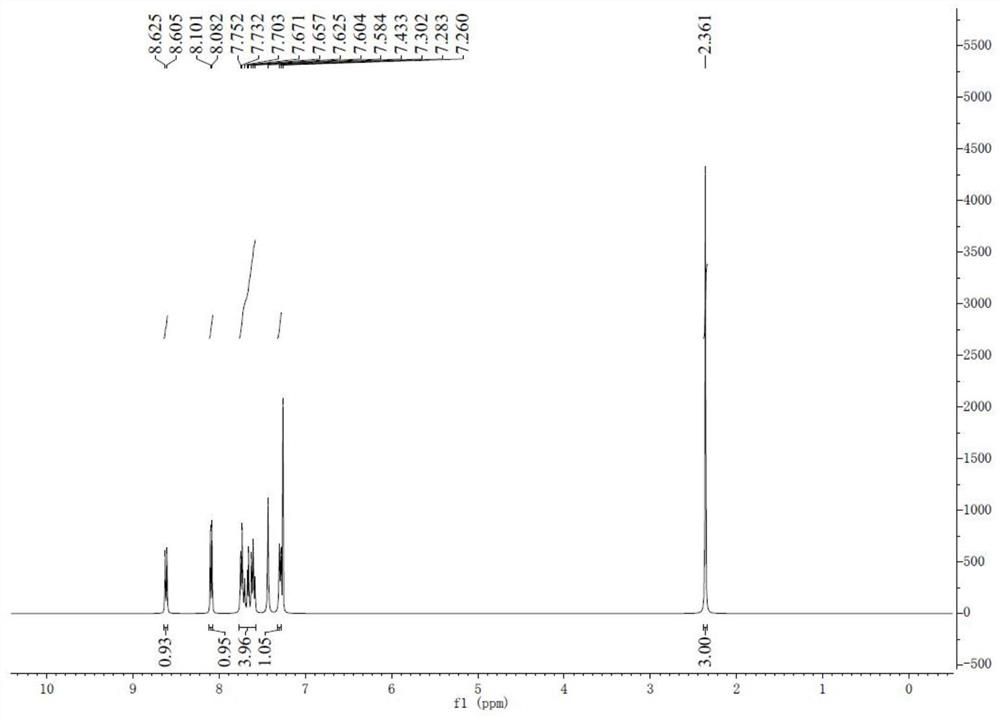
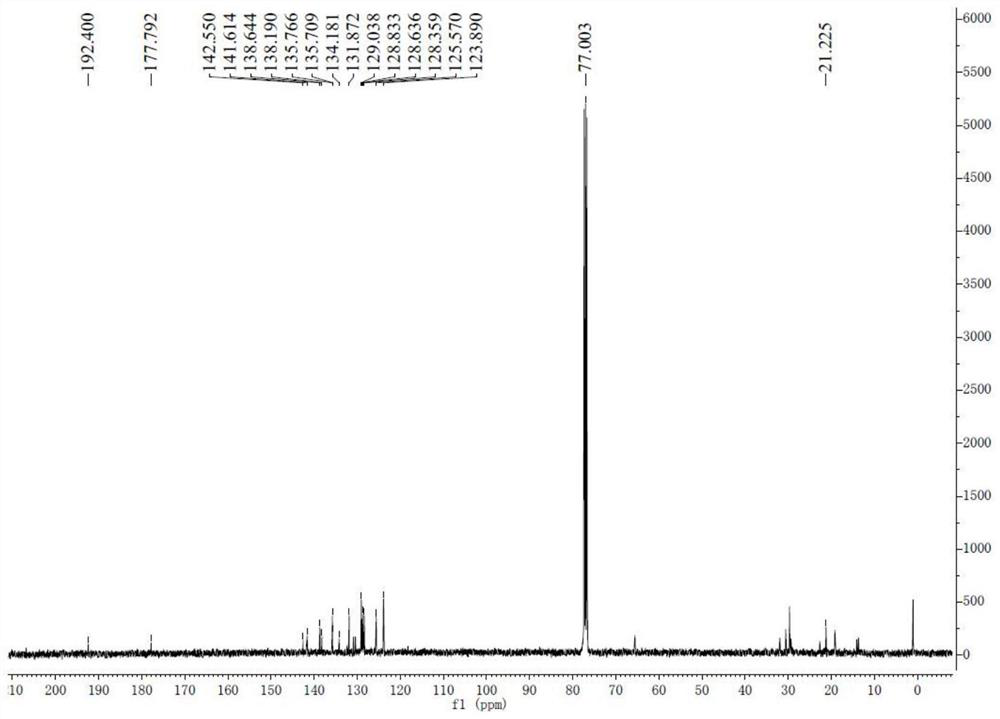
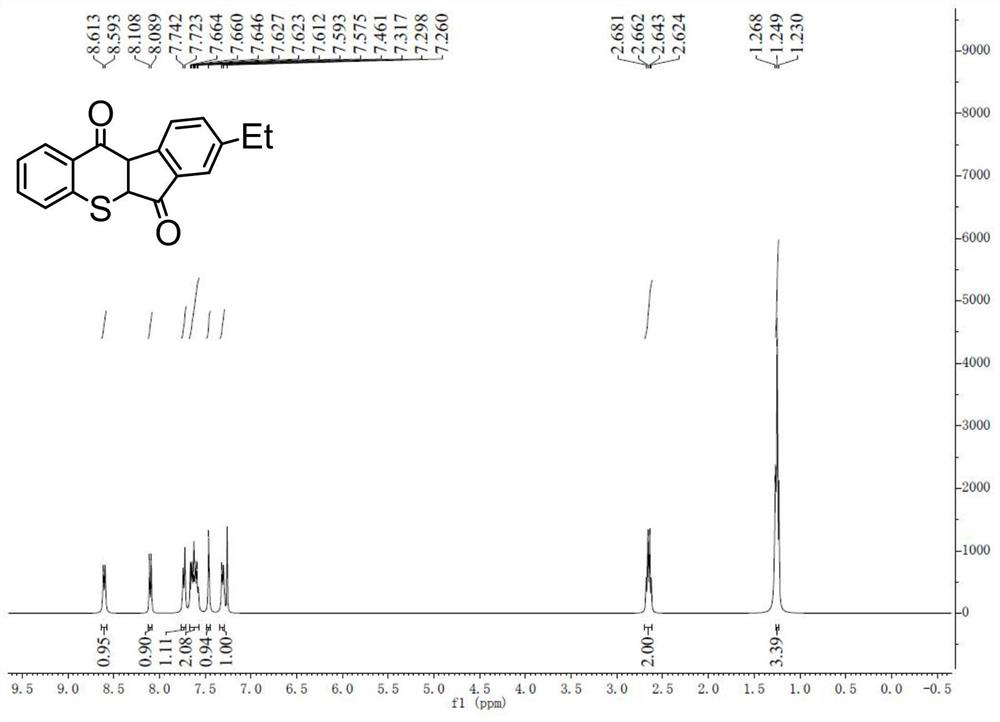
[0045] The synthesis of 8-ethylbenzothiopyrandione is the same as in Example 1.
[0046] 1 H NMR (CDCl 3 / TMS) δ: 8.60 (d, J = 7.8Hz, 1H), 8.10 (d, J = 7.5Hz, 1H), 7.73 (d, J = 7.8Hz, 1H), 7.62 (ddd, J = 19.2, 11.0 ,4.2Hz,2H),7.46(s,1H),7.31(d,J=7.5Hz,1H),2.65(q,J=7.5Hz,2H),1.25(s,3H).
[0047] 13 C NMR (CDCl 3 )δ:192.49,177.84,145.09,145.09,142.68,141.85,138.22,135.72,134.79,134.20,131.91,130.49,129.06,128.67,128.40,124.49,124.02,125.6,767.3
Embodiment 3
[0049] The synthesis of 10-methylbenzothiopyrandione is the same as in Example 1.
[0050] 1 H NMR (CDCl 3 / TMS)δ:8.56(d,J=8.5Hz,1H),7.74–7.57(m,4H),7.52(d,J=7.1Hz,1H),7.33(d,J=7.7Hz,1H), 7.16(t,J=7.4Hz,1H),2.80(s,3H).
[0051] 13 C NMR (CDCl 3 )δ: 192.45, 177.31, 144.10, 142.58, 141.07, 140.31, 135.39, 134.61, 131.82, 131.30, 129.64, 128.40, 128.09, 122.81, 77.36, 23.34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com