Formation method of lithium ion battery
A lithium-ion battery and formation method technology, which is applied in the field of formation of lithium-ion batteries, can solve the problems of difficult to balance high-temperature and low-temperature performance, the use temperature window is not very wide, and the high-temperature capacity retention rate is poor, so as to shorten the formation time, Effects of improving cycle performance and simplifying chemical synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
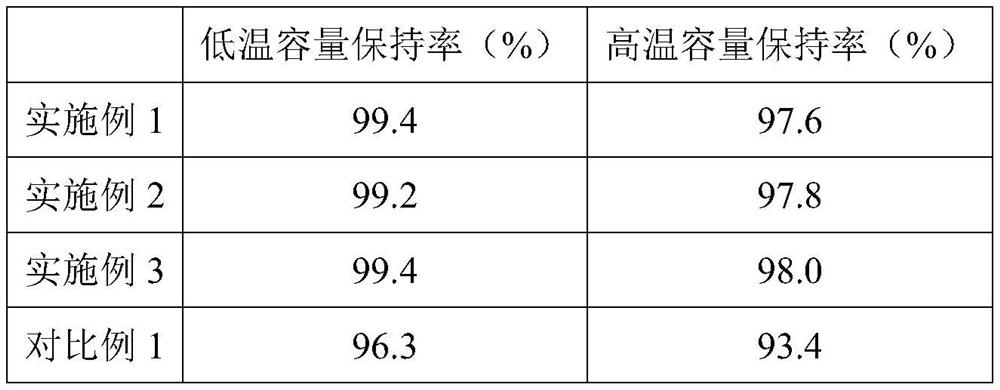
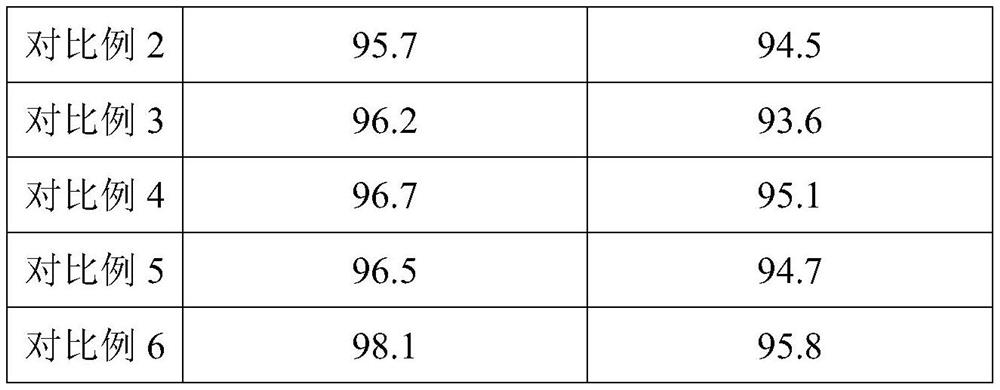
Embodiment 1
[0027] 1) The electrolyte is injected into the battery to be injected, and the electrolyte includes linear carbonates and cyclic carbonates, electrolyte salts and additives; the linear carbonates account for 50% of the total electrolyte volume, and the rest are cyclic carbonates. Carbonate; wherein the additives include fluoroethylene carbonate, divinyl sulfone and α, α-dimethyl-γ-butyrolactone, wherein the volume concentration of the additive satisfies the following relationship, (volume of fluoroethylene carbonate concentration+α, the volume concentration of α-dimethyl-γ-butyrolactone)*the volume of linear carbonate=k*divinyl sulfone*the volume of cyclic carbonate, wherein k=1.62; the fluorinated The volume concentration of ethylene carbonate: α, the volume concentration of α-dimethyl-γ-butyrolactone=1:1.6, the volume concentration of described divinyl sulfone is 2.4%, the volume concentration of fluoroethylene carbonate is 1.50%, the volume concentration of α,α-dimethyl-γ-b...
Embodiment 2
[0035] 1) Inject the electrolyte into the battery to be injected, the electrolyte includes linear carbonate and cyclic carbonate, electrolyte salt and additives; the linear carbonate accounts for 55% of the total electrolyte volume, and the rest is ring Carbonate; wherein the additives include fluoroethylene carbonate, divinyl sulfone and α, α-dimethyl-γ-butyrolactone, wherein the volume concentration of the additive satisfies the following relationship, (volume of fluoroethylene carbonate concentration+α, the volume concentration of α-dimethyl-γ-butyrolactone)*the volume of linear carbonate=k*divinyl sulfone*the volume of cyclic carbonate, wherein k=1.65; the fluorinated The volume concentration of ethylene carbonate: α, the volume concentration of α-dimethyl-γ-butyrolactone=1:1.6, the volume concentration of described divinyl sulfone is 2.6%, the volume concentration of fluoroethylene carbonate is 1.35%, the volume concentration of α,α-dimethyl-γ-butyrolactone is 2.16%;
[...
Embodiment 3
[0043] 1) The electrolyte is injected into the battery to be injected, and the electrolyte includes linear carbonates and cyclic carbonates, electrolyte salts and additives; the linear carbonates account for 50% of the total electrolyte volume, and the rest are cyclic carbonates. Carbonate; wherein the additives include fluoroethylene carbonate, divinyl sulfone and α, α-dimethyl-γ-butyrolactone, wherein the volume concentration of the additive satisfies the following relationship, (volume of fluoroethylene carbonate concentration+α, the volume concentration of α-dimethyl-γ-butyrolactone)*the volume of linear carbonate=k*divinyl sulfone*the volume of cyclic carbonate, wherein k=1.64; the fluorinated The volume concentration of ethylene carbonate: α, the volume concentration of α-dimethyl-γ-butyrolactone=1:1.6, the volume concentration of described divinyl sulfone is 2.5%, the volume concentration of fluoroethylene carbonate is 1.58%, the volume concentration of α,α-dimethyl-γ-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com