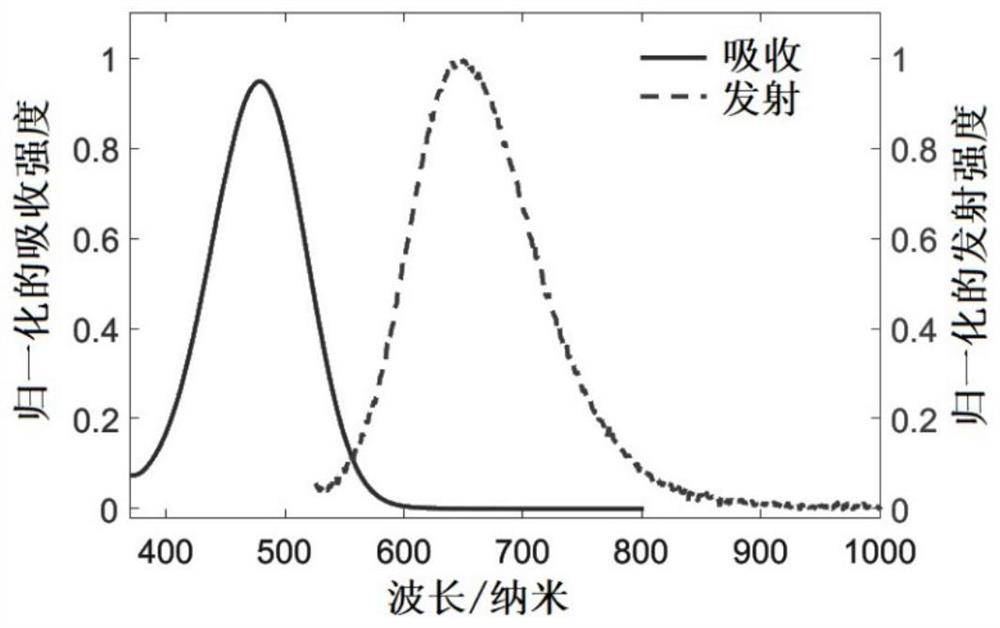
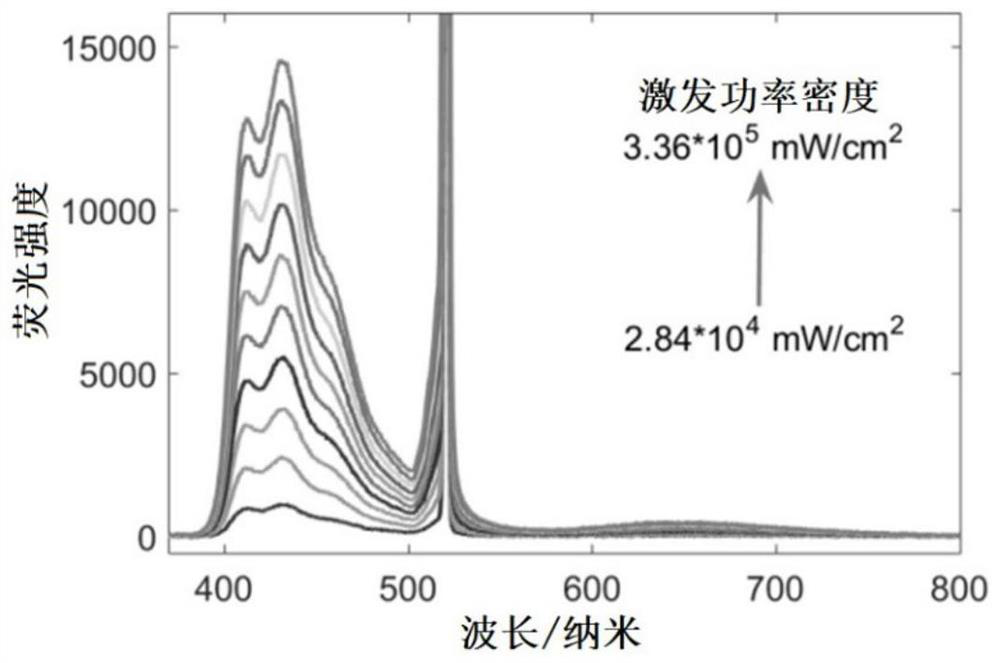
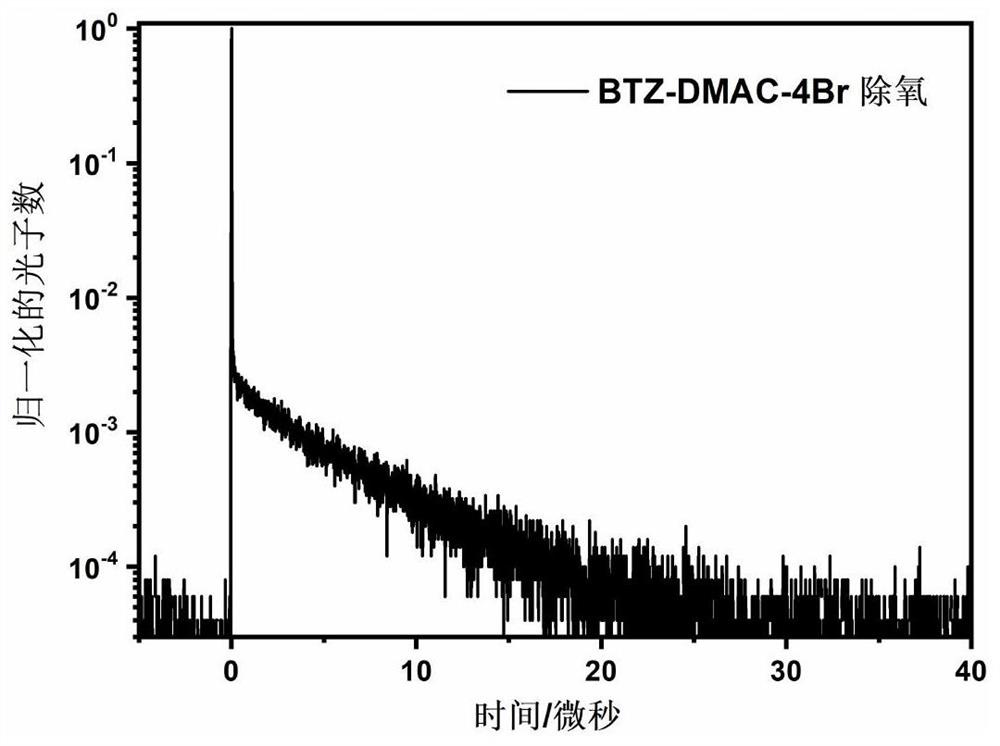
Compound and preparation method thereof, and triplet-triplet annihilation up-conversion system
A triplet annihilation and compound technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve the problems of low upconversion quantum yield and high excitation threshold, so as to improve the upconversion quantum efficiency and increase the minimum triplet Effect of state exciton concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of the compound BTZ-DMAc-2Cl with the structure represented by formula (I).
[0052] (1) intermediate: the synthesis of the compound BTZ-DMAc of the structure shown in formula (II)
[0053]
[0054] Add BTZ-Br (500mg, 2.34mmol), 9,9-dimethylacridine (DMAc, 735mg, 3.51mmol), palladium acetate (78mg, 0.35mmol) and tri-tert-butylphosphine tetrafluoro Borate (305mg, 1.05mmol), sodium tert-butoxide (808mg, 8.42mmol), and 30mL of toluene treated with water and oxygen removal were added under argon atmosphere, and reacted at 120°C for 48 hours. Cool to room temperature, pour the reaction solution into 50 mL of ice water, extract three times with dichloromethane, concentrate the organic phase, and separate by column chromatography (n-hexane:dichloromethane, v:v, 3:1) to obtain 601 mg of red powder, product rate of 75%. MS (EI) m / z: 342.12.
[0055] (2) Synthesis of BTZ-DMAc-2Cl of structure shown in formula (I)
[0056]
[0057] In a 100 mL roun...
Embodiment 2
[0058] Example 2: Preparation of the compound BTZ-2DMAc-4Cl with the structure shown in formula (I).
[0059] (1) intermediate: the synthesis of the compound BTZ-2DMAc of the structure shown in formula (II)
[0060]
[0061] Add BTZ-Br (500mg, 1.71mmol), 9,9-dimethylacridine (DMAc, 787mg, 3.76mmol), palladium acetate (57mg, 0.26mmol) and tri-tert-butylphosphine tetrafluoro Borate (223mg, 0.77mmol), sodium tert-butoxide (591mg, 6.16mmol), add 22mL of toluene treated with water and oxygen removal under argon atmosphere, and react at 120°C for 48 hours. Cool to room temperature, pour the reaction solution into 50 mL of ice water, extract three times with dichloromethane, concentrate the organic phase, and separate by column chromatography (n-hexane:dichloromethane, v:v, 2:1) to obtain 658 mg of red powder, product rate of 70%. MS (EI) m / z: 549.22.
[0062] (2) Synthesis of the compound BTZ-2DMAc-2Cl of the structure shown in formula (I)
[0063]
[0064] In a 100 mL rou...
Embodiment 3
[0065] Example 3: Preparation of the compound BTZ-DMAc-2Br with the structure shown in formula (I).
[0066] (1) intermediate: the synthesis of the compound BTDZ-DMAc of the structure shown in formula (II)
[0067]
[0068] Add BTDZ-Br (500mg, 2.32mmol), 9,9-dimethylacridine (DMAc, 728mg, 3.48mmol), palladium acetate (78mg, 0.34mmol) and tri-tert-butylphosphine tetrafluoro Borate (303mg, 1.04mmol), sodium tert-butoxide (802mg, 8.35mmol), and 30mL of toluene treated with water and oxygen removal were added under argon atmosphere, and reacted at 120°C for 48 hours. Cool to room temperature, pour the reaction solution into 50 mL of ice water, extract three times with dichloromethane, concentrate the organic phase, and separate by column chromatography (n-hexane:dichloromethane, v:v, 3:1) to obtain 574 mg of red powder, product rate of 72%. MS (EI) m / z: 343.11.
[0069] (2) Synthesis of the compound BTDZ-DMAc-2Br of the structure shown in formula (I)
[0070]
[0071] In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com