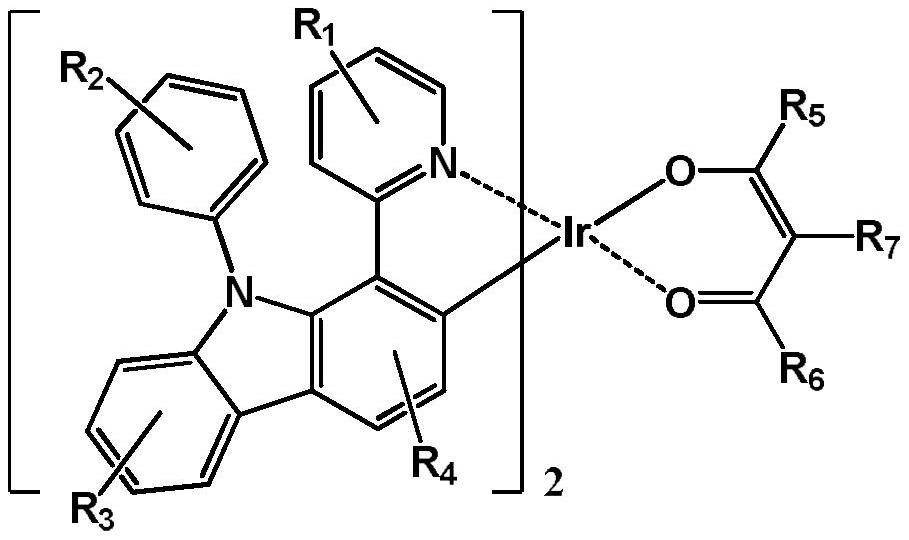
Iridium complex with main ligand containing carbazolyl and application
A technology of iridium complexes and alkyl groups, which is applied in the direction of indium organic compounds, platinum group organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., and can solve the problems of increasing the non-radiative transition rate of molecules and unfavorable luminescent processes , to achieve the effect of improving device efficiency, high external quantum efficiency, and excellent device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 prepares the iridium complex AG02 of the present invention
[0035]
[0036] Preparation of compound L1-1 in the above route: 1-bromo-9H-carbazole (3.00g, 12.24mmol), iodobenzene (3.00g, 14.71mmo), K 2 CO 3 (2.53g, 18.36mmol) and DMPU (10 drops) were added into a sealed pressure-resistant tube, and heated at 200°C for 18h. After cooling to ambient temperature, the mixture was filtered, washing with dichloromethane. The filtrate was concentrated, and the residue was purified by silica gel column chromatography (pure PE eluent) to obtain 3.50 g of compound L1-1 (89% yield).
[0037] Preparation of compound L1-2 in the above route: add n-BuLi (4.78mL, 12.00mmol, 2.50M hexane solution) dropwise to L1-1 (3.50g, 10.90mmol, 1.00equiv.) in anhydrous tetrahydrofuran (60mL ) solution, a pale yellow solution was obtained at -78°C. After stirring for 1 h, trimethyl borate (1.70 g, 16.35 mmol, 1.50 equiv.) was added. The resulting solution was stirred at -78°C f...
Embodiment 2
[0043] Embodiment 2 prepares the iridium complex AG26 of the present invention
[0044]
[0045] Similarly, referring to the synthesis scheme of Example 1 above, the iridium complex AG26 can be prepared by replacing the corresponding raw materials and following a certain mass ratio of the substances. 1 H NMR (500MHz, CD 2 Cl 2 )δ8.52(d, J=5.9Hz, 2H), 7.96(d, J=7.6Hz, 2H), 7.71(s, 2H), 7.62(d, J=8.2Hz, 4H), 7.57–7.49( m,5H),7.36(dd,J=8.8,5.5Hz,6H),7.28(t,J=7.3Hz,3H),7.14(d,J=5.8Hz,2H),6.09(d,J=7.9 Hz,2H),5.68(s,1H),1.04(s,18H). 13 C NMR (126MHz, CD 2 Cl 2 )δ195.19,167.47,154.22,148.29,142.16,141.94,140.02,137.36,137.09,129.96,128.91,126.85,126.57,125.51,125.09,122.01,121.37,121.32,118.85,118.00,115.58,110.79,90.14,53.88,53.67 ,53.45,53.23,53.02,41.15,27.96.HRMS(MALDI-TOF,m / z):[M] + calcd for C59H47F6IrN4O2, 1050.323; found, 1050.666.
Embodiment 3
[0046] Embodiment 3 prepares the iridium complex AG50 of the present invention
[0047]
[0048] Similarly, referring to the synthesis schemes of the above-mentioned examples 1 and 2, the iridium complex AG50 can be prepared by replacing the corresponding raw materials and following a certain mass ratio of the substances. 1 H NMR (500MHz, CD 2 Cl 2 )δ8.32(d, J=5.4Hz, 2H), 7.94(d, J=7.5Hz, 2H), 7.70–7.58(m, 7H), 7.45(d, J=7.8Hz, 6H), 7.29( ddd, J=23.4, 15.4, 7.3Hz, 7H), 7.06(d, J=5.2Hz, 2H), 6.10(d, J=7.8Hz, 2H), 5.66(s, 1H), 1.06(s, 18H ),0.25(s,18H). 13 C NMR (126MHz, CD 2 Cl 2 )δ194.39,164.77,153.59,150.11,146.12,142.48,142.21,139.15,130.76,129.64,127.08,126.93,126.19,124.91,124.69,124.62,121.67,121.16,119.65,118.67,110.75,89.75,53.88,53.67,53.45 ,53.24,53.02,41.13,28.03,-1.69.HRMS(MALDI-TOF,m / z):[M] + calcd for C 63 h 65 IrN 4 o 2 Si 2 , 1058.424; found, 1059.346.
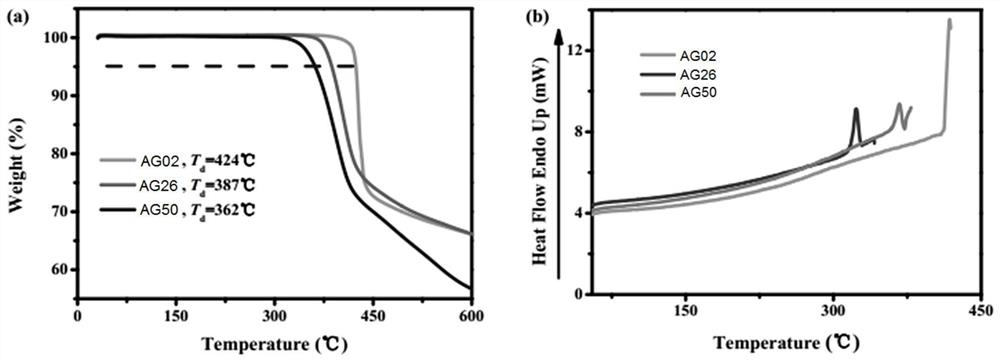
[0049] The iridium complexes AG02, AG26, AG50 thermal analysis spectrum of the present invent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com