Surface defect passivated metal halide perovskite nanocrystals, their preparation and applications
A metal halide, perovskite technology, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc. High dynamic instability and other problems, to achieve the effect of improving photoluminescence quantum yield, solving non-radiation loss, and benefiting performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] In some embodiments, the preparation method includes the following steps:
[0041] (1) Mix the halides, metal halides, and surface active ligands of the A-position monovalent cations in an organic solvent to obtain a metal halide perovskite nanocrystal precursor solution; wherein the A-position monovalent cations The halide is excessive relative to the metal halide, that is, the molar amount of the halide of the A-position monovalent cation is greater than the molar amount of the metal halide;
[0042] (2) mixing the precursor solution obtained in step (1) with a poor solvent to obtain the crude liquid of the metal halide perovskite nanocrystal;
[0043] (3) Mix the crude liquid obtained in step (2) with the medium solvent to obtain the precipitate, add the dispersion liquid, collect the stably dispersed supernatant to obtain the colloidal solution of the metal halide perovskite nanocrystal.
[0044] In some embodiments, the halide of the A-position monovalent cation i...
Embodiment 1
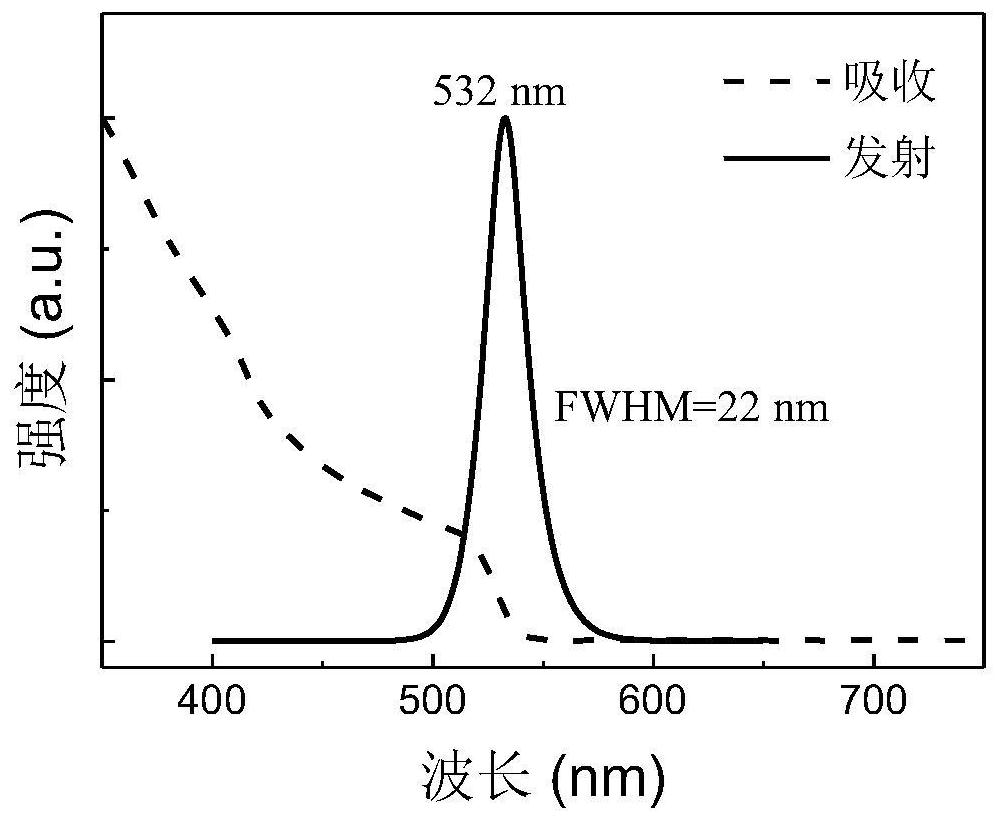
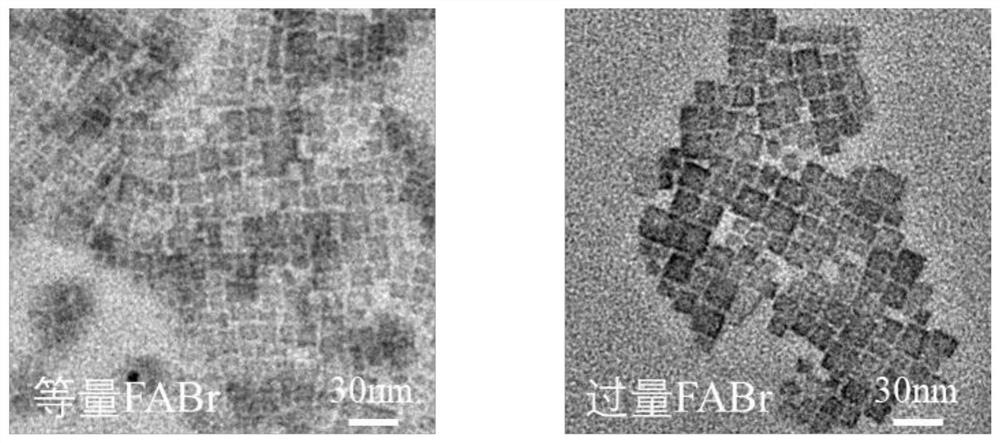
[0059] This embodiment provides a method to prepare FAPbBr using excess FABr 3 The method of NCs and its application in electroluminescent diodes include the following steps:
[0060] Preparation of FAPbBr 3 NCs solution: accurate weighing of PbBr with electronic balance 2 (0.0367g, 0.1mmol) and 0.22mmolFABr (FABr:PbBr 2 =2.2:1), add 0.5mL DMF solvent, place on a magnetic stirrer and stir until the raw materials are completely dissolved. Then, 250 μL of oleic acid and 25 μL of n-octylamine were added and mixed to obtain a precursor solution. The precursor solution was quickly added dropwise per 100 μL into a vigorously stirred 8 mL chloroform solution with a pipette gun, and green-emitting NCs were formed immediately. After stirring for 35 s, the stirring was stopped to obtain the crude NCs solution. Add 3 mL of acetonitrile to the obtained NCs crude solution, and then centrifuge at 11000 r.p.m. for 5 minutes. Discard the supernatant and disperse the pellet in 4 mL of ...
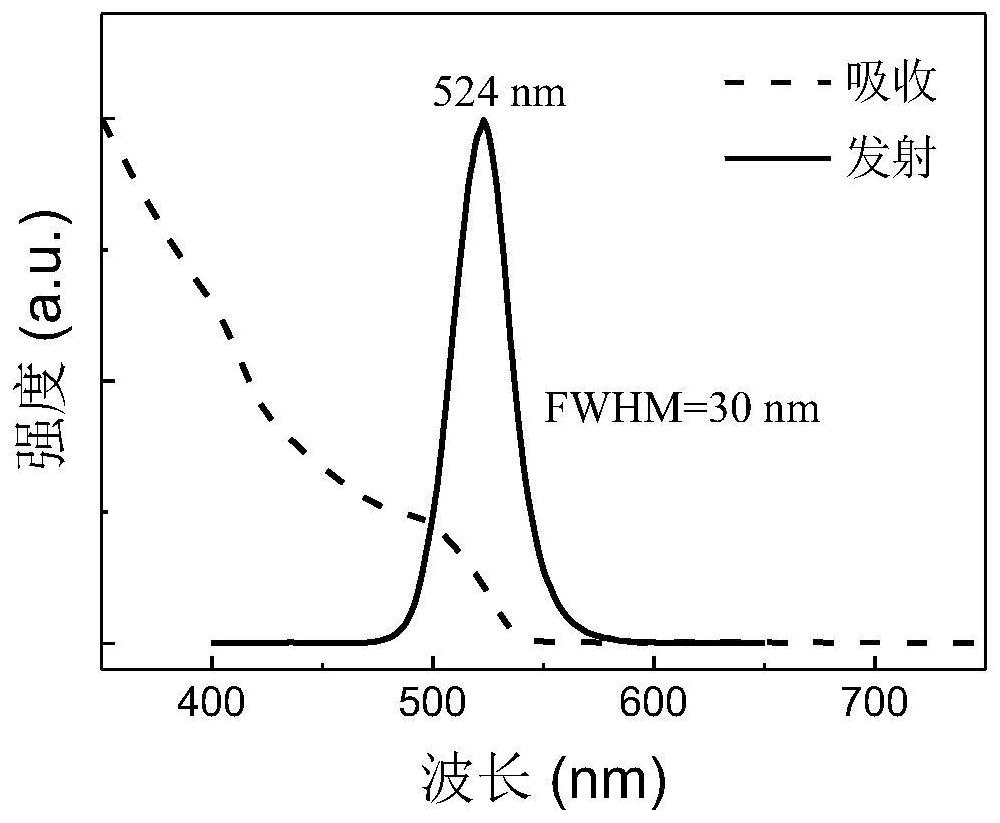
Embodiment 2
[0076] Preparation of CsPbBr 3 NCs solution: accurate weighing of PbBr with electronic balance 2 (0.0367g, 0.1mmol) and 0.2mmol CsBr, add 1mL DMSO solvent, place on a magnetic stirrer and stir until the raw materials are completely dissolved. Then, 250 μL oleic acid and 125 μL oleylamine were both added, and mixed to obtain a precursor solution. The precursor solution was quickly added dropwise per 100 μL into the vigorously stirred 8 mL toluene solution with a pipette gun, and green-emitting NCs were formed immediately. After stirring for 35 s, the stirring was stopped to obtain the crude NCs solution. Add 27 mL of ethyl acetate to the obtained NCs crude solution, and then centrifuge at 11000 r.p.m. for 5 minutes. Discard the supernatant and disperse the pellet in 4 mL of octane. Finally, the octane dispersion was centrifuged at 6000 r.p.m. for 3 minutes to remove large perovskite particles, and then the stable dispersed supernatant was collected and filtered through a 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com