Methods of treating malignant lymphoproliferative disorders
A technology for lymphoid hyperplasia and malignancy, which is used in pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1 - Viability and Proliferation Assay (MTS Assay)
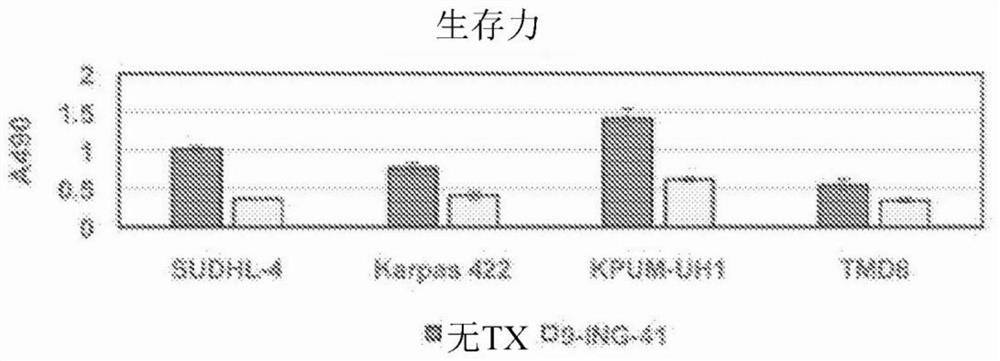
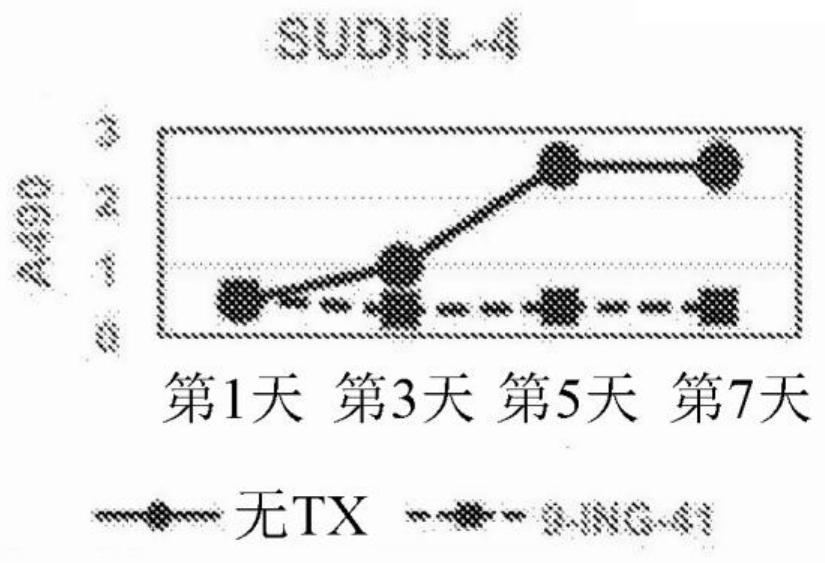
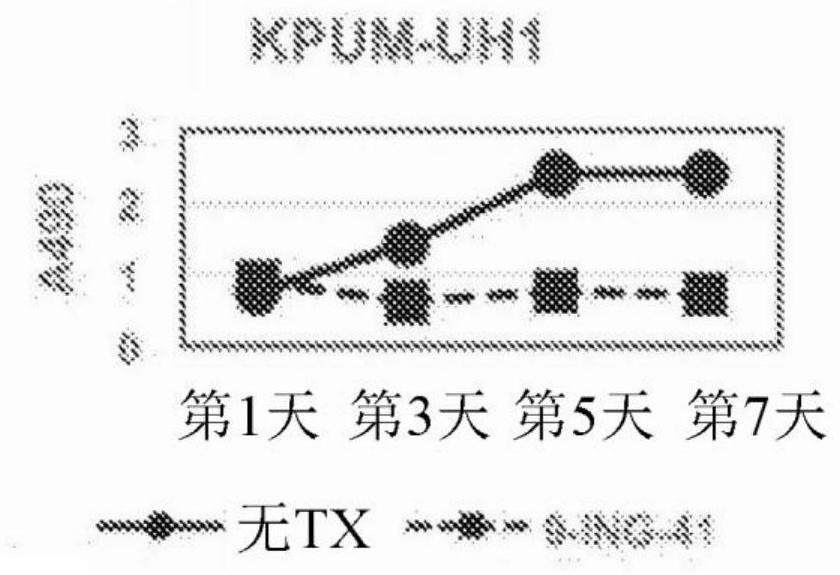
[0123] After treating cells with different concentrations of 9-ING-41, Promega CellTiter The AQueous One Solution Cell Proliferation Assay (MTS) measures cell viability at day 3 and proliferation over the course of 7 days according to the manufacturer's instructions. At the end of the treatment, add 20 µL of reagent per well in the 96-well plate and incubate at 37 °C for 2-4 h. Absorbance at 490 nm (A490) was measured using a Powerwave XS plate reader (Biotek).
[0124] All lymphoma cell lines used in this study expressed active GSK-3β. SUDHL-4, KPUM-UH1, Karpas 422, or TMD8 lymphoma cells were plated and cell numbers were measured on days 1, 3, 5, and 7 using the MTS assay. See Figure 1. After 1 μM 9-ING-41 treatment, the cell viability on day 3 ( Figure 1A ) was reduced by 40-70% (p Figures 1B-1E ), the proliferation on day 7 was less than 30% relative to the control (p<0.05). Cell viability of lymph...
Embodiment 3-W
[0130] Example 3 - Western blot analysis
[0131]c-MYC levels in KPUM-UH1 cells were analyzed by Western blot for 9-ING-41 alone and 9-ING-41 in combination with venetoclax or BAY-1143572. Approximately 10 million cells were spun at 200 rcf for 5 min and rinsed once with PBS before lysing in 50 μl Millipore Milliplex MAP lysis buffer supplemented with protease and phosphatase inhibitors (Roche). Each well was loaded with denatured protein in 4X sample buffer supplemented with β-mercaptoethanol (Bio-Rad). Bio-Rad stain-free Criterion 4-20% precast gels were used. After running the gel at 140 volts for 90 min, the stain-free technique was activated using a Bio-Rad gel imager to visualize the level of total protein loaded in the gel. The protein was then transferred to the membrane using a nitrocellulose turbo-transfer kit and system (Bio-Rad), and the membrane was blocked using 5% w / v dry milk in Tris-buffered saline-0.1% Tween 20 (TBS-T)1 Hour. Membranes were then incubat...
Embodiment 4
[0132] Example 4-Luminex analysis
[0133] NF-κB associated with 1 μM 9-ING-41 treatment for 48 hours compared to untreated controls was determined using the FLEXMAP 3D instrument according to the manufacturer's instructions [MILLIPLEXMAP NF-κB Signal Transduction Magnetic Bead Kit 6-plex Kit, EMD Millipore, Analytes: c-MYC, FADD (Ser194), IκBα (Ser32), IKKα / β (Ser177 / Ser181), NF-κB (Ser536), TNFR1], DNA damage [MILLIPLEX MAP DNA damage / Genotoxic Magnetic Bead Set, EMD Millipore, Analytes: ATR(Total), Chk1(Ser345), Chk2(Thr68), H2A.X(Ser139), MDM2(Total), p21(Total), p53(Ser15)] and Apoptotic Pathway [Bio-plex pro RBM Apoptosis Group 2 and 3, Bio-Rad, Analytes: Bad, Bax / Bcl-2dimer, Bcl-xL, Bim, Mcl-1, Active Caspase 3, Bcl-xL / Bak dimer, Mcl-1 / Bak dimer, survivin] signaling changes. Cells were lysed in MILLIPLEX MAP Lysis Buffer supplemented with Protease Inhibitor Cocktail (Sigma) and Phosphatase Inhibitor Cocktails 2 and 3 (Sigma), and after BCA protein determination, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com