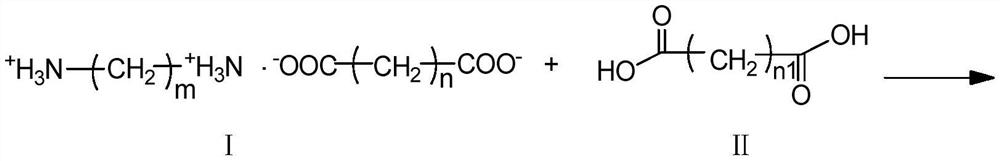Preparation method of carboxyl-terminated diamide
A technology of carboxyl-terminated diamides and diamine diacid salts, which is applied in the preparation of carboxyl-terminated diamides and the field of preparation of carboxyl-terminated diamides. It can solve the problems of high production costs and complicated process, and achieve slow activity and simple reaction process. , Improve the effect of hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
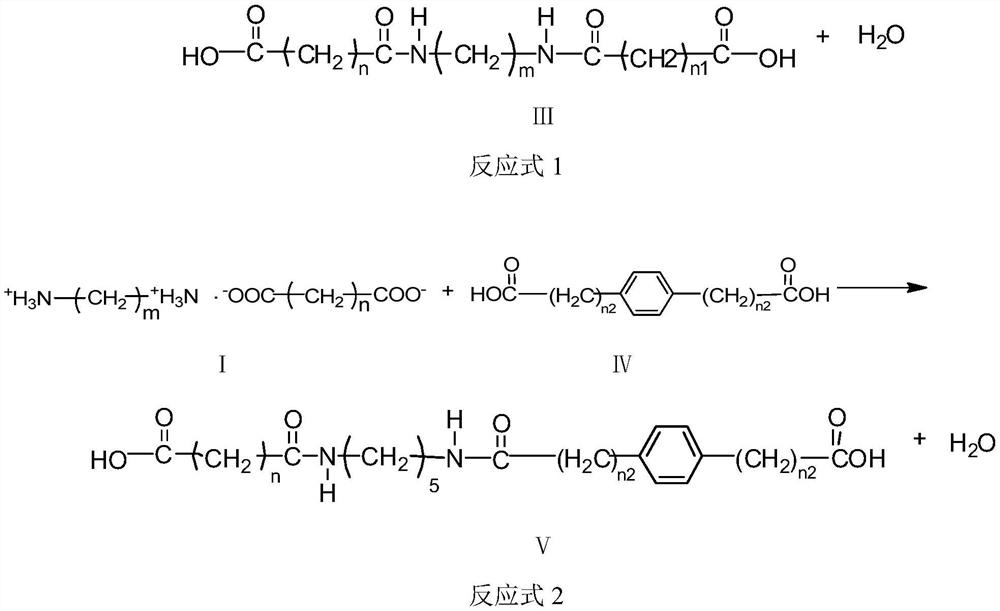
[0032] A kind of preparation method of carboxyl-terminated diamide, such as reaction formula 1 or reaction formula 2, diamine diacid salt (structural formula is I), dibasic acid (structural formula is II or IV) and non-polar organic solvent (toluene, One or more of xylene and trimethylbenzene) are mixed and reacted, and the generated water is removed by azeotropic distillation while reacting to obtain a carboxyl-terminated diamide; wherein, the molar ratio of diamine diacid salt to dibasic acid is 1 :1~2, the ratio of the volume of the nonpolar organic solvent to the mass of the diamine diacid salt is 1~10:1 (mL / g), the reaction temperature is 10~30°C higher than the boiling point of the nonpolar organic solvent, and the reaction The time is 12-20 hours, and the azeotropic distillation adopts an oil-water separation device. The temperature of the azeotropic distillation is higher than the boiling point of the non-polar organic solvent, and the time of the azeotropic distillatio...
Embodiment 1
[0036] A kind of preparation method of carboxyl-terminated diamide, diamine diacid salt (structural formula is ), dibasic acid (structural formula is ) mixed with a non-polar organic solvent (xylene) to react, carry out azeotropic distillation to remove the generated water while reacting, and obtain the carboxyl-terminated diamide; wherein, the molar ratio of the diamine diacid salt to the dibasic acid is 1:1.5, the ratio of the volume of non-polar organic solvent to the mass of diamine diacid salt is 6:1 (mL / g), the reaction temperature is 160°C, the reaction time is 18h, and the azeotropic distillation adopts an oil-water separation device. The temperature of azeotropic distillation is 160°C, and the time of azeotropic distillation is 18h;
[0037] After the reaction is completed, use an alcoholic organic solvent (ethanol) for purification. The specific process is: firstly mix the reacted mixture with an alcoholic organic solvent at a temperature of 65°C, cool to normal te...
Embodiment 2
[0047] A kind of preparation method of carboxyl-terminated diamide, diamine diacid salt (structural formula is ), dibasic acid (structural formula is ) mixed with a non-polar organic solvent (toluene) to react, and carry out azeotropic distillation to remove the generated water while reacting to obtain a carboxyl-terminated diamide; wherein, the molar ratio of diamine diacid salt to dibasic acid is 1 : 1.4, the ratio of the volume of the non-polar organic solvent to the mass of the diamine diacid salt is 6:1 (mL / g), the reaction temperature is 150°C, the reaction time is 16h, and the azeotropic distillation adopts an oil-water separation device. The temperature of boiling distillation is 150°C, and the time of azeotropic distillation is 16h;
[0048] After the reaction is completed, use an alcoholic organic solvent (ethanol) for purification. The specific process is: firstly mix the reacted mixture with an alcoholic organic solvent at a temperature of 65°C, cool to normal t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com