Oxazolyl steroid derivative as well as synthesis method and application thereof
A technology of oxazolyl steroids and derivatives, which is applied in the field of drug synthesis, can solve the problems of yield and quality loss, and achieve the effect of high production yield and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In the following, the technical solution of the present invention will be described in conjunction with examples, but the present invention is not limited to the following examples.
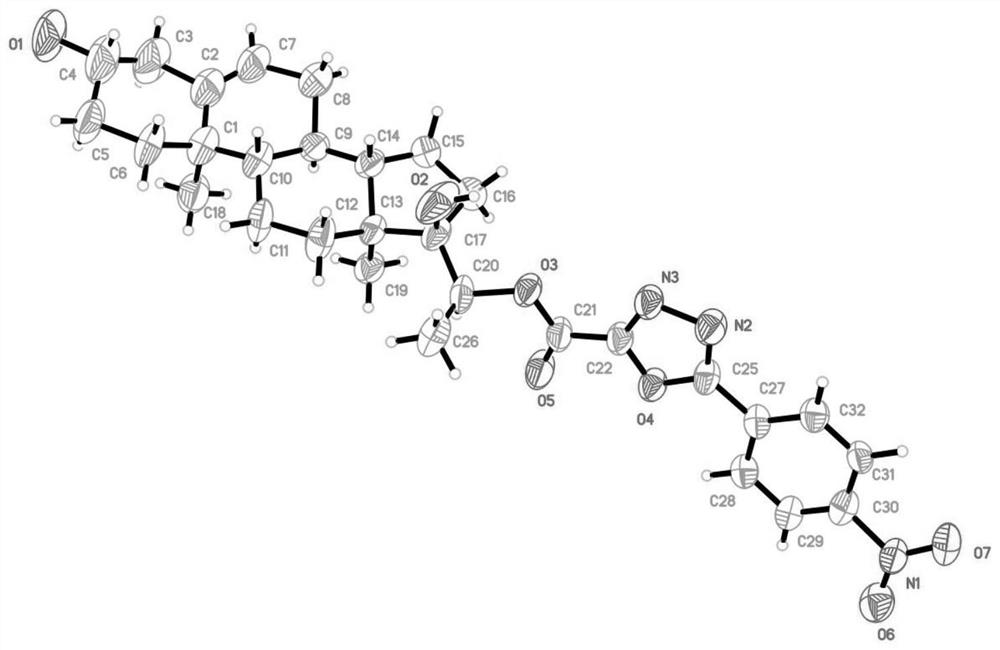
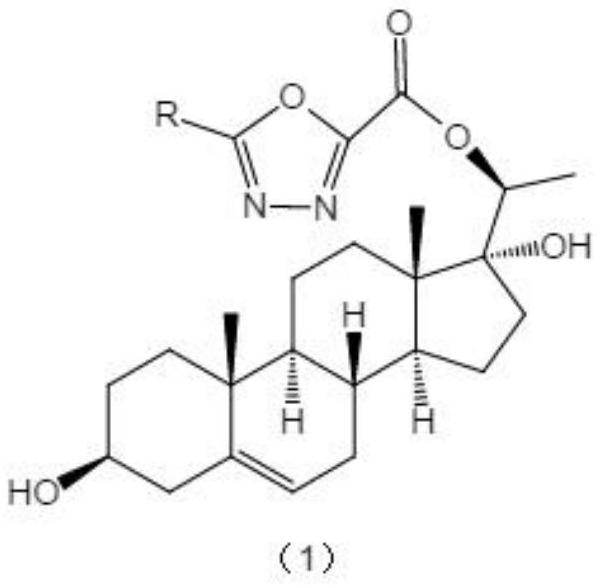
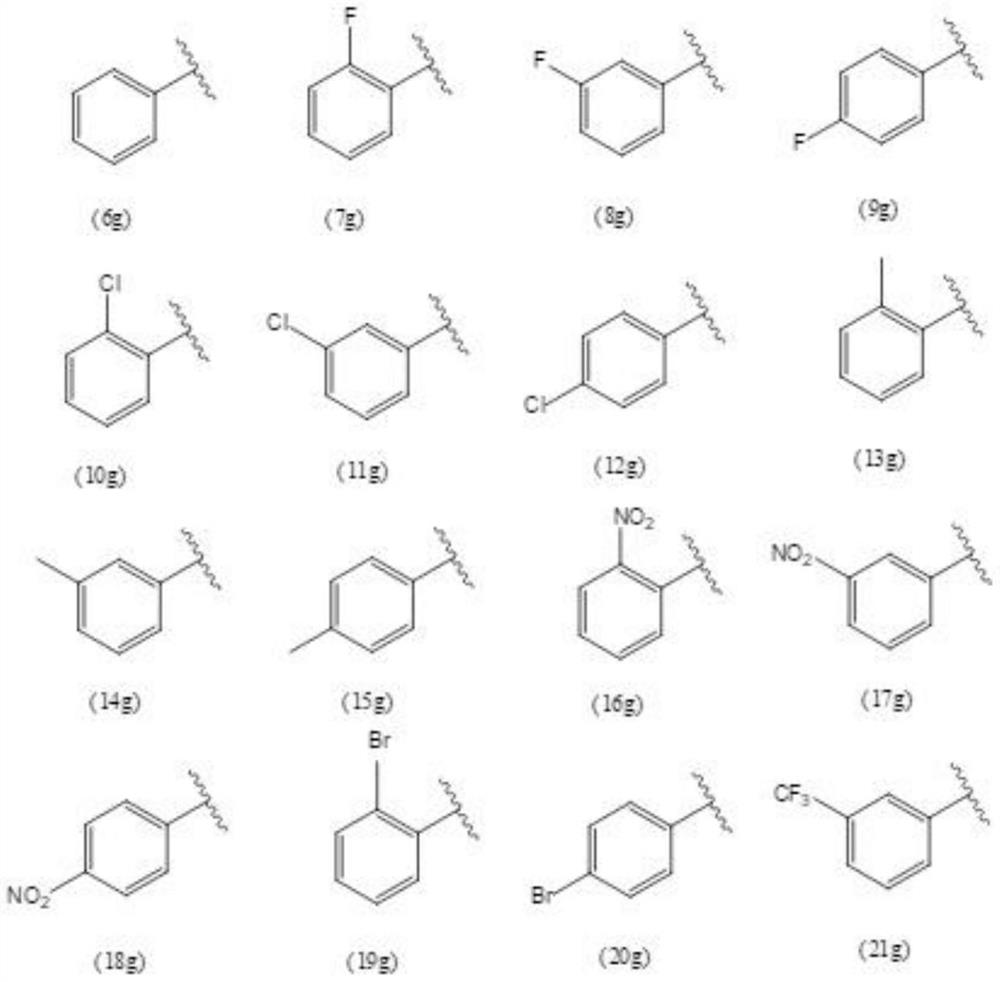
[0029] Synthesis and preparation of the oxazolyl steroid derivatives of the present invention. Its synthesis idea can be divided into three parts: the first part, first react dehydroepiandrosterone 1 with N,N-dimethylformamide, tert-butyldimethylchlorosilane to protect the three-position hydroxyl of 1, and then tetrahydrofuran As a solvent, ethyltriphenylphosphine bromide and potassium tert-butoxide were added to obtain compound 3, and finally asymmetric dihydroxylation reaction occurred under the catalysis of AD-mix-bata to obtain compound 4; the second part, with various acid chlorides As the reaction raw material, a hydrazinolysis reaction occurs in the case of ethanol as a solvent to obtain 6c-31c, and then dichloromethane is used as a solvent to close the ring in the presence of triet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com