Application of human sDR5-Fc recombinant fusion protein in preparation of drug for preventing and treating acute kidney injury
An acute kidney injury and fusion protein technology, applied in the field of recombinant proteins, can solve the problems of transmitting apoptosis signals, etc., and achieve broad application prospects, unique effects, and enhanced curative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Design and recombination of recombinant human DR5-Fc expression sequence
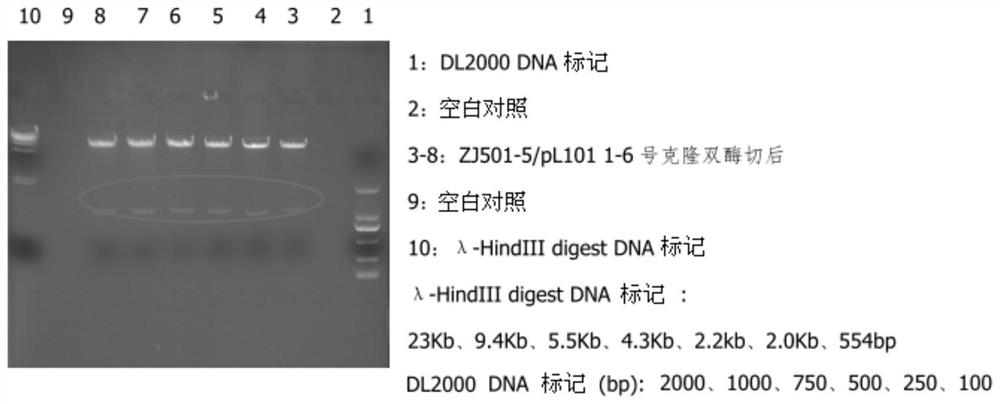
[0047] After long-term accumulation of experience, the inventor constructed a recombinant fusion protein and fused human DR5 with Fc in various ways. The results of mass spectrometry analysis showed that most of the target proteins were cleaved at the N-terminal 11 amino acids (ITQQDLAPQQR). In order to express N The target protein with a relatively uniform terminal was finally screened to remove the intermediate connection sequence and 18 amino acids at the N-terminus on the basis of the commonly used signal peptide of the fusion protein, and named it sDR5-Fc (ZJ501-5). The plasmid was transiently transfected, and the expression supernatant was purified for mass spectrometry and activity analysis.
[0048] DNA sequence of ZJ501-5:
[0049] atgggtgtactgctcacacagaggacgctgctcagtctggtccttgcactcctgtttccaagcatggcgagcatgtccagcccctcagagggattgtgtccacctggacaccatatctcagaagacggtagagattgcatctcctg...
Embodiment 2
[0053] Example 2: Expression of Human sDR5-Fc Recombinant Protein and Detection of Physicochemical Properties
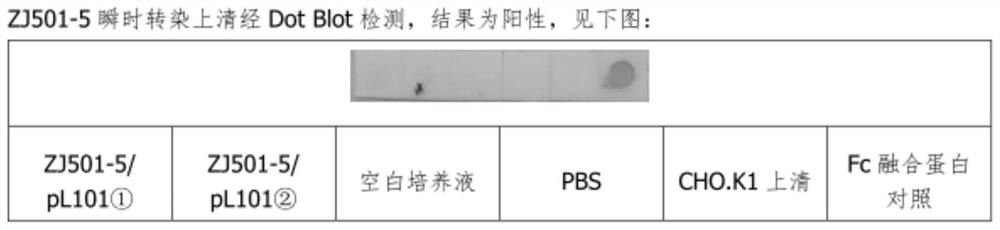
[0054] The sDR5-Fc / pL101 plasmid was transiently transfected into CHO.K1 cells with Lipofectamine2000, and the supernatant was collected for Dot Blot detection after 48 hours. The result was positive, see figure 2 .
[0055] To recover CHO.K1-S cells, press 5×10 5 / ml 10ml after resuspension, use FreeStyle TM CHO Expression Medium serum-free medium, and add Glutamine at a final concentration of 8mmol / L for culture, placed at 37°C in 8% CO 2 Shake the incubator at 120rpm for cultivation. When the number of cells>1×10 6 Subculture and add liquid to 30ml, maintain cell number 2-5×10 5 / ml, each subsequent passage and maintain the density to 2-5×10 5 / ml, CHO.K1-S needs to be subcultured more than three times.
[0056] The day before transfection, adjust the cell density to 5-6×10 5 / ml 100ml, placed at 37°C 8% CO 2 Shake the incubator at 120rpm for culture, a...
Embodiment 3
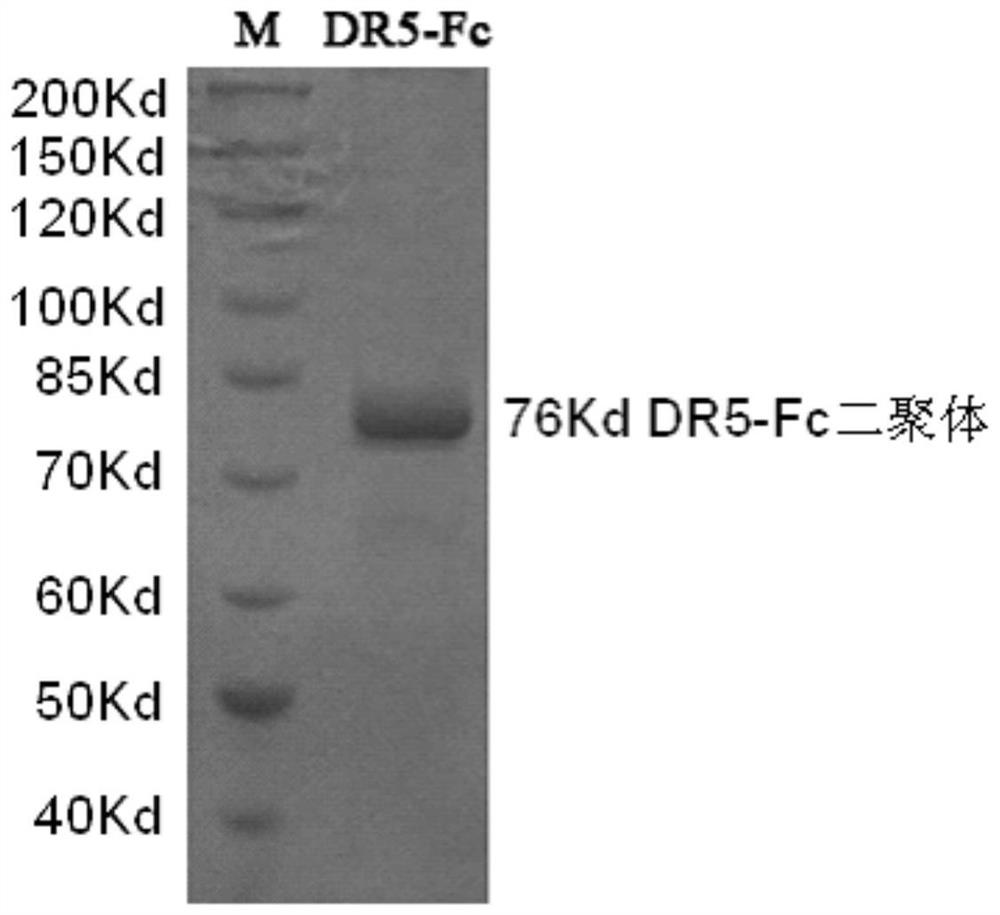
[0081] Example 3: Identification of biological activity of ZJ501-5
[0082] (1) Establish a commercial Trail killing activity detection method
[0083] Collect the Jurkat cells in the logarithmic growth phase, resuspend the cells with 10% FCS RPMI-1640 / DMEM after counting, and adjust the cell density to 8*10 4 / ml, 100ul / well was added to a 96-well cell culture plate, and placed in a 37°C, 8% carbon dioxide incubator for 20-24 hours.
[0084] Resuspend the commercial Trail in the above-mentioned complete culture solution containing actinomycin D (final concentration is 0.03ug / ml) at a final concentration of 500-1000ng / ml, and use the complete culture solution containing actinomycin D for 2 times Specific dilution commercial Trail, a total of 15-20 concentration gradients. The diluted sample was added to a 96-well cell culture plate at 100ul / well, and placed in an incubator for 18-22 hours. Add 20ul / well of freshly prepared 20:1 mixed MTS / PMS chromogenic solution, continue t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com