Application of fusion protein in preparation of drug for treating hepatitis C
A fusion protein and hepatitis C technology, applied in the biological field, can solve the problems of side effects, serious drug-resistant drug interactions, etc., achieve the effect of reducing liver cell apoptosis, reducing liver inflammation, and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
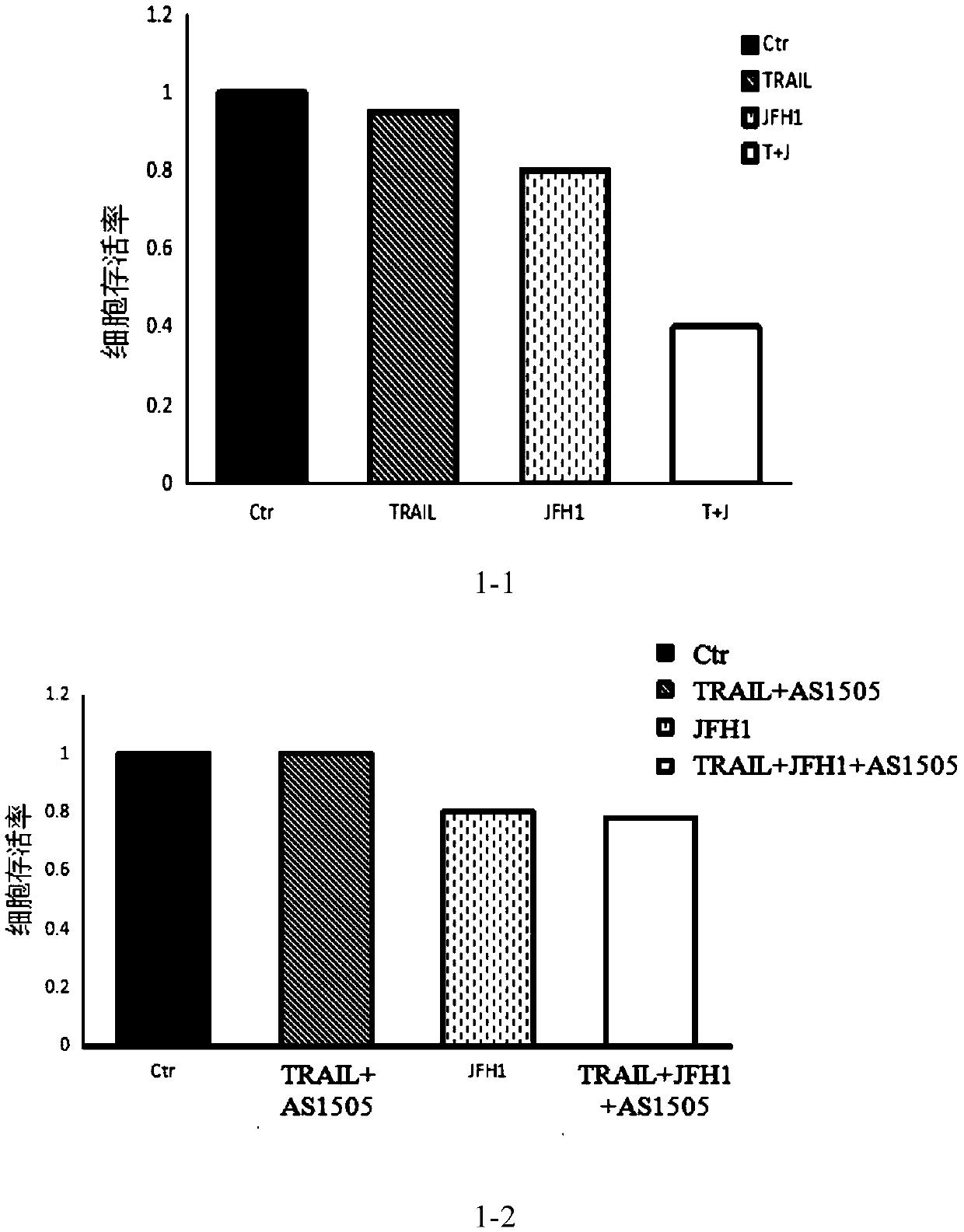
[0030] Embodiment 1: the therapeutic effect of fusion protein in the cell model of infectious HCV
[0031] The fusion protein (code name: AS1505) is expressed in mouse CHO cells, specifically the amino acid sequence shown in SEQ ID NO: 1:
[0032] SSPSEGLCPPGHHISEDGRDCISCKYGQDYSTHWNDLLFCLRCTRCDSGEVELSPCTTTRNTVCQCEEGTFREEDSPEMCRKCRTGCPRGMVKVGDCTPWSDIECVHKEESKYGPPCPSCPAPEFLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQ EGNVFSCSVMHEALHNHYTQKSLSLSLGK
[0033] After the FL-JFH1 plasmid was amplified and identified by enzyme digestion, it was amplified and purified in vitro, and then transferred to Huh7.5.1 by electroporation. The transfected Huh7.5.1 was conventionally subcultured, and the HCV titer was measured by fluorescent quantitative PCR, and the HCV titer was plotted Curves over time, HCV (JFH1) supernatants were prepared in large quantitie...
Embodiment 2
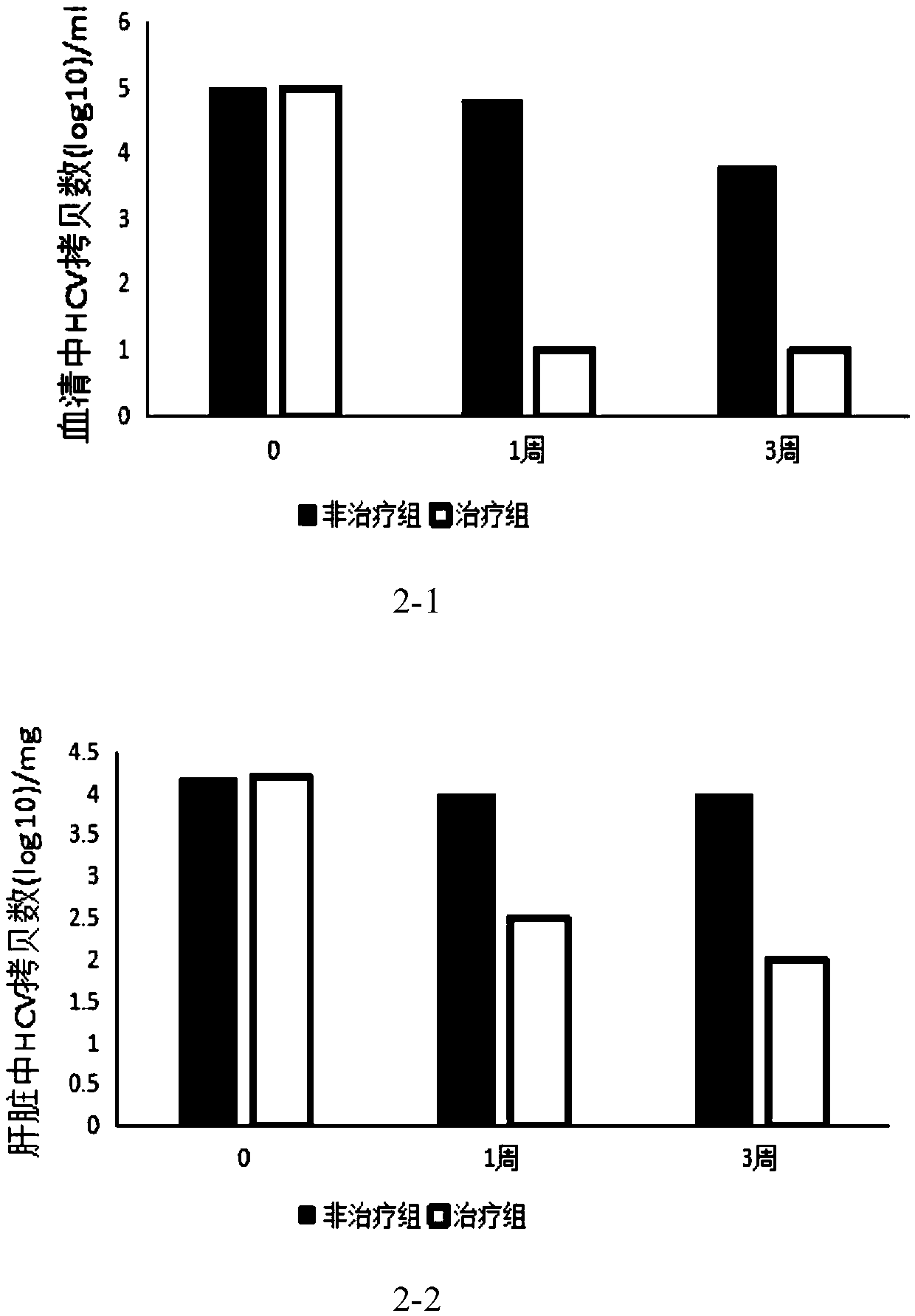
[0037] Embodiment 2: the therapeutic effect of fusion protein in HCV infection mouse model
[0038] CD81 and occludin (OCLN) double transgenic mice were used and divided into treatment group and non-treatment group. HCV was injected through the tail vein, and the injection time was controlled at 1-2min. Drug treatment was started for the treatment group one week after infection, and the fusion protein was administered for 3 weeks, administered twice a week, each intraperitoneal injection of 10mg / ml, the dose 10mg / kg, 1 week and 3 weeks after treatment, the serum and liver tissue of mice in treatment group and non-treatment group were collected. qRT-PCR method was used to detect the copy number of HCV RNA in serum and hepatocytes.
[0039] See the test results figure 2 .
[0040] Depend on figure 2 It can be seen that the non-treatment group is the copy number of HCV in the serum and the replication in the liver within three weeks after the HCV-infected mouse model is not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com