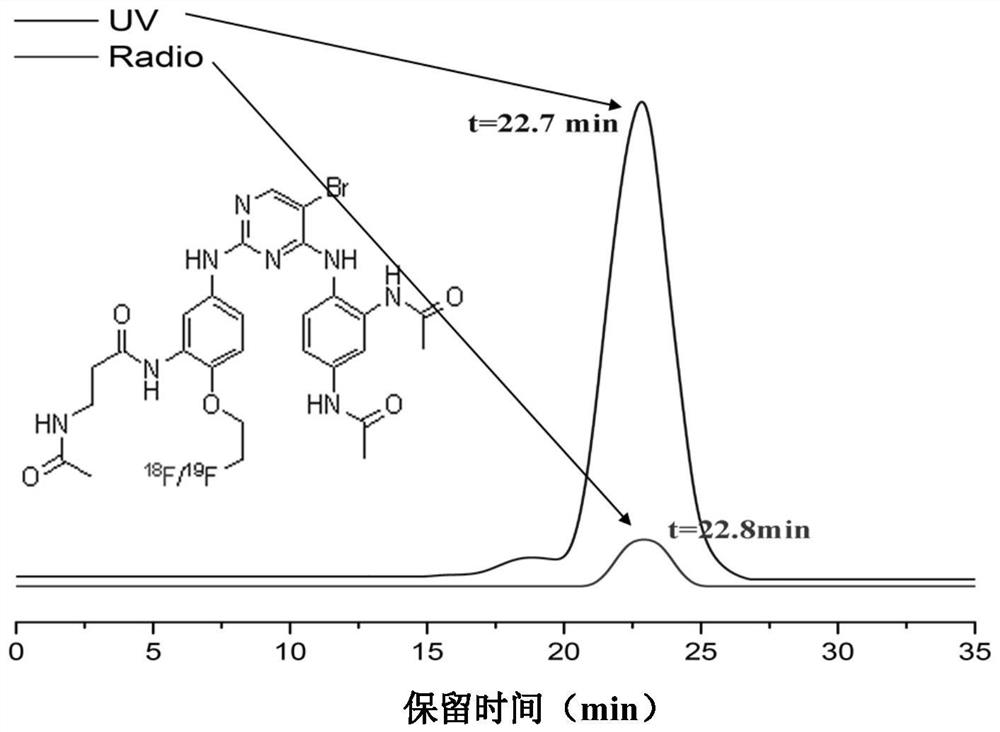
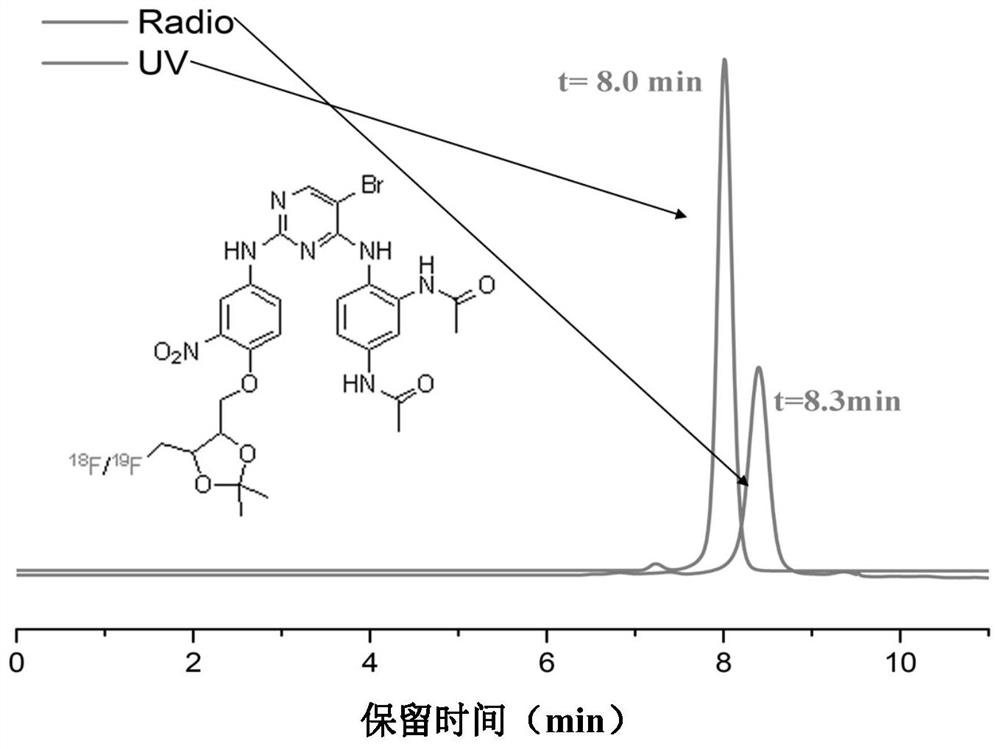
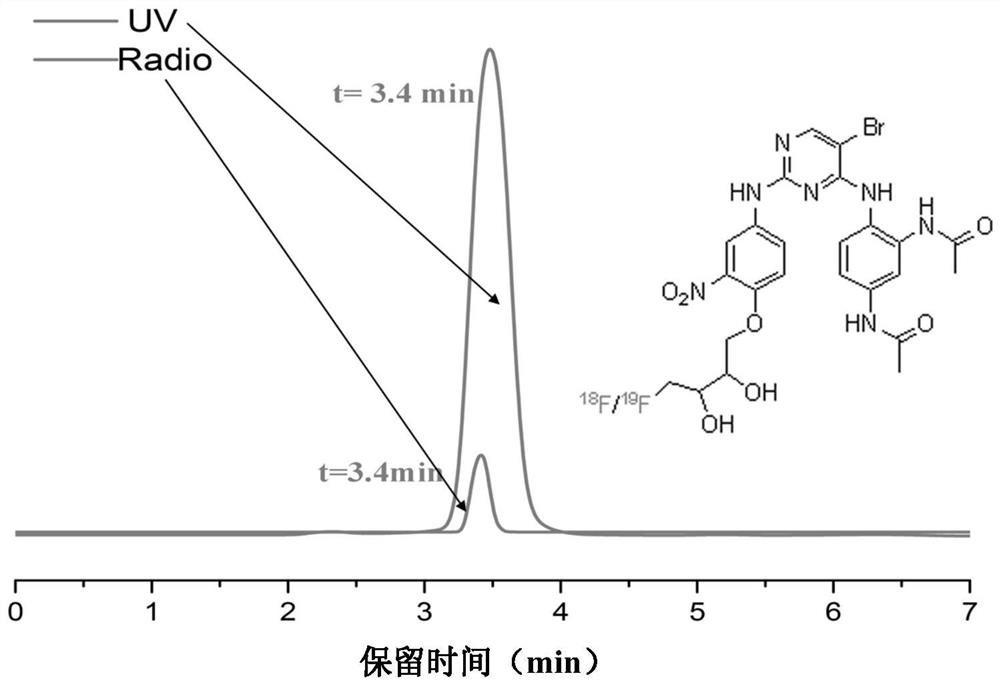
Focal adhesion kinase (FAK)-targeting compound and preparation method and application thereof
A technology of compound and precursor compound, applied in the field of compound, can solve the problem that tumor suppressor activity needs to be improved, and achieve the effect of good affinity, high selectivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] The second aspect of the application provides the preparation method of the compound described in the first aspect of the application, which includes:
[0075] 1) make the compound of formula (II):
[0076]
[0077] With the compound of general formula (Ⅲ):
[0078] R'-NH 2 (Ⅲ),
[0079] In an organic solvent, at 90-110 ° C, the compound of general formula (IV) is synthesized by catalyzing p-toluenesulfonic acid:
[0080]
[0081] Wherein, R' is selected from
[0082] R' 1 from-NO 2 ,
[0083] 3) to Substitution compound -OH, obtain the compound of general formula (Ⅴ) or general formula (Ⅵ):
[0084]
[0085] 4) to Substitution compound -NO in 2 , obtain the compound of general formula (VII):
[0086]
[0087] Among them, R' 2 selected from -OH,
[0088] 5) -NO in the compound of general formula (Ⅳ)-(Ⅶ) 2 At least one of , -OH or -OTs is replaced by a fluorine-containing compound to obtain a compound of formula (I):
[0089] ...
preparation example 1
[0116] Preparation Example 1. Organic Synthesis of Compound 79 and Compound 80
[0117] The synthetic route is as follows:
[0118]
[0119] To 5-bromo-2,4-dichloropyrimidine (compound 60) (25.0 g, 111.1 mmol, 1 equiv) in THF (200 mL), add 2,4-dinitroaniline (compound 61) (24.4 g, 133.3mmol, 1.2equiv) in THF (200mL) and potassium carbonate (18.4g, 133.3mmol, 1.2equiv), heated to 70°C overnight. After the reaction, potassium carbonate was filtered out, and potassium carbonate was washed with ethyl acetate (50 mL*3 times). The organic phase was collected, the filtrate was concentrated by rotary evaporation, and separated on a medium-pressure preparative Flash silica gel column (petroleum ether / ethyl acetate=10 / 1 to 5 / 1) to obtain compound 62 (21.9 g, yellow solid, yield 50.8%);
[0120] 1 H NMR (400MHz, CDCl 3 ,δppm): 11.33(s,1H), 9.32(d,J=9.2Hz,1H), 9.21(s,1H), 8.59(s,2H).
[0121] To a solution of compound 62 (20.0 g, 53.7 mmol, 1 equiv) in tetrahydrofuran (100 mL) and m...
preparation example 2
[0137] Preparation Example 2. Organic Synthesis of Compound 83 and Compound 84
[0138] The synthetic route is as follows:
[0139]
[0140] Compound 79 was obtained by referring to the method of Preparation Example 1;
[0141] To compound 79 (500 mg, 0.97 mmol, 1 equiv) in DMF (6 mL), add potassium carbonate (200 mg, 1.45 mmol, 1.5 equiv), add compound 81 (911 mg, 1.94 mmol, 2 equiv) in DMF (5 mL), The temperature was raised to 60°C for 3h. The reaction solution was concentrated by rotary evaporation, and separated on a medium-pressure Flash silica gel column (dichloromethane / methanol=20 / 1 to 5 / 1) to obtain compound 82 (505 mg, yellow solid, yield 64.1%);
[0142] 1 H NMR (600MHz, DMSO-d 6 ,δppm):10.02(s,1H),9.99(s,1H),9.43(s,1H),8.20(s,1H),8.16(s,1H),7.97(s,1H),7.76-7.73( m,3H),7.62(s,1H),7.49(d,J=8.2Hz,1H),7.39-7.38(m,3H),7.06(d,J=9.5Hz,1H),4.30(dd,J =2.0Hz, 10.6Hz, 1H), 4.16-4.11(m, 4H), 4.05-4.02(m, 1H), 2.32(s, 3H), 2.04(d, J=13.9Hz, 6H), 1.25(d ,J=13.4Hz,6H). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com