Crystal of upatinib intermediate compound and preparation method thereof
A compound and crystal technology, applied in the field of drug synthesis, can solve the problems of unfavorable scale-up production, large amount of solvents, cumbersome operation, etc., and achieve the effect of controllable process, low cost and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
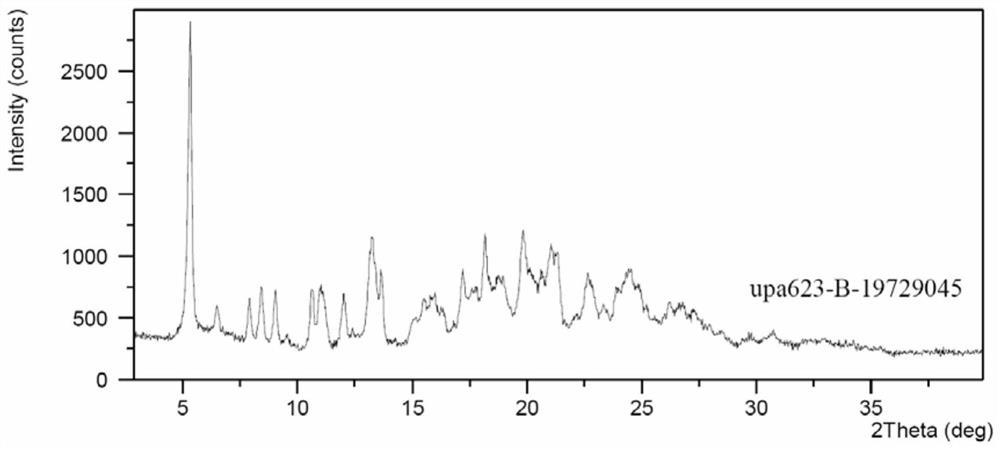
[0072] The preparation method of the oxalate salt form C of compound I:
[0073] Dissolve 400 mg of compound I in 4 mL of isopropyl acetate, slowly add 94 mg / 4 mL of oxalic acid dihydrate in isopropyl acetate dropwise at 20-30°C, stir at room temperature for 2 h, filter and drain to obtain a solid 204 mg, the sampling test HPLC purity was 99.82%.
[0074] 2theta d interval Relative Strength% 9.26 9.55 40.54 10.04 8.81 17.63 12.62 7.01 24.42 15.87 5.58 24.87 16.18 5.48 33.76 16.66 5.32 26.40 16.91 5.24 67.12 18.10 4.90 19.03 20.14 4.41 29.72 20.75 4.28 24.13 21.57 4.12 29.38 22.56 3.94 100.00 25.21 3.53 18.46 25.39 3.51 27.92
Embodiment 2
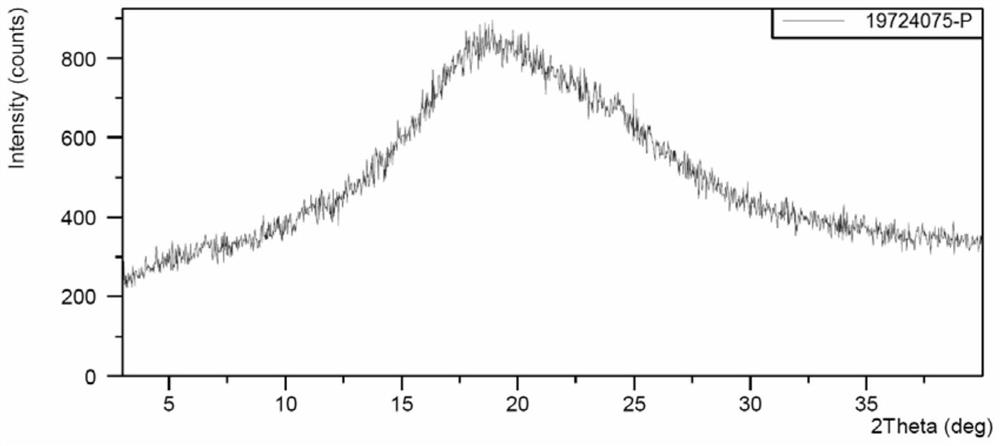
[0076] Amorphous preparation method of Compound I (refer to patent WO2011068881, Example AA.1.160, page 363 and its related reference examples; WO2019016745, Example 27&28, pages 40-41):
[0077]
[0078] Dissolve 2.0 g of compound 3 in 10 mL of 1,4-dioxane, slowly add 6 mL of 33% HBr / CH 3 COOH, stirred at 20-30°C for 2 hours, the reaction was complete, added 10 mL of water to quench the reaction, added 10 mL of toluene, stirred and separated, continued to add 10 mL of toluene to the water phase, stirred and separated to obtain the water phase, adjusted the pH of the system to 7 with NaOH ~8, add 20 mL of dichloromethane, stir and separate to obtain an organic phase, add 10 mL of Brine to the organic phase for washing, and obtain a dichloromethane solution of compound 4, which is designated as solution A. Take another 25mL three-necked flask, add 895mg N,N-carbonyldiimidazole, add 10mL dichloromethane, add 547mg 2,2,2-trifluoroethylamine at 20-30°C, stir for 1 hour, record ...
Embodiment 3
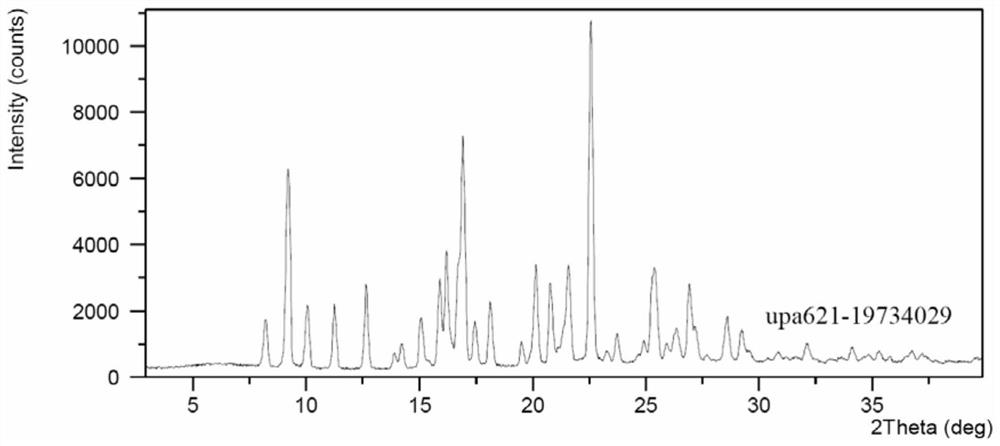
[0080] The preparation method of the crystal form A of compound I:
[0081] Dissolve 1 g of compound I in 5 mL of isopropyl acetate, heat to 50-60 °C, add 100 mg of compound I seed crystals, the system slowly precipitates more solids, slowly cool down to 20-30 °C, and stir for 2 h , Suction filtration to obtain 650 mg of compound I with a purity of 99.74%.
[0082] HNMR data:
[0083] 1 H NMR (400 MHz, d-DMSO) δ 8.77 (m, 1H), 8.05-8.03 (m, 2H), 7.99-7.97 (m,1H), 7.59 (s, 1H), 7.46-7.43 (m, 3H ), 6.96-6.93 (m, 1H), 4.34-4.29 (m, 1H),3.88-3.65 (m, 5H), 3.27-3.23 (m, 1H), 2.35 (s, 3H), 1.04-0.99 (m , 1H), 0.82-0.77 (m, 1H), 0.63-0.60 (m, 3H).
[0084] 2theta d interval Relative Strength% 5.32 16.60 100.00 7.88 11.21 15.15 8.42 10.51 19.19 9.03 9.79 18.93 10.58 8.36 17.93 10.96 8.07 19.27 12.01 7.37 16.33 13.20 6.71 34.73 13.64 6.49 22.90 15.96 5.55 15.91 17.18 5.16 22.68 18.15 4.89 34.91 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com