Method for establishing induced pluripotent stem cells from human amniotic epithelial cells
A technology of pluripotent stem cells and epithelial cells, applied in the field of reprogramming human amniotic epithelial cells into induced pluripotent stem cells, which can solve the problems of cumbersome process and achieve the effect of sharp edges and large nuclear-to-cytoplasmic ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
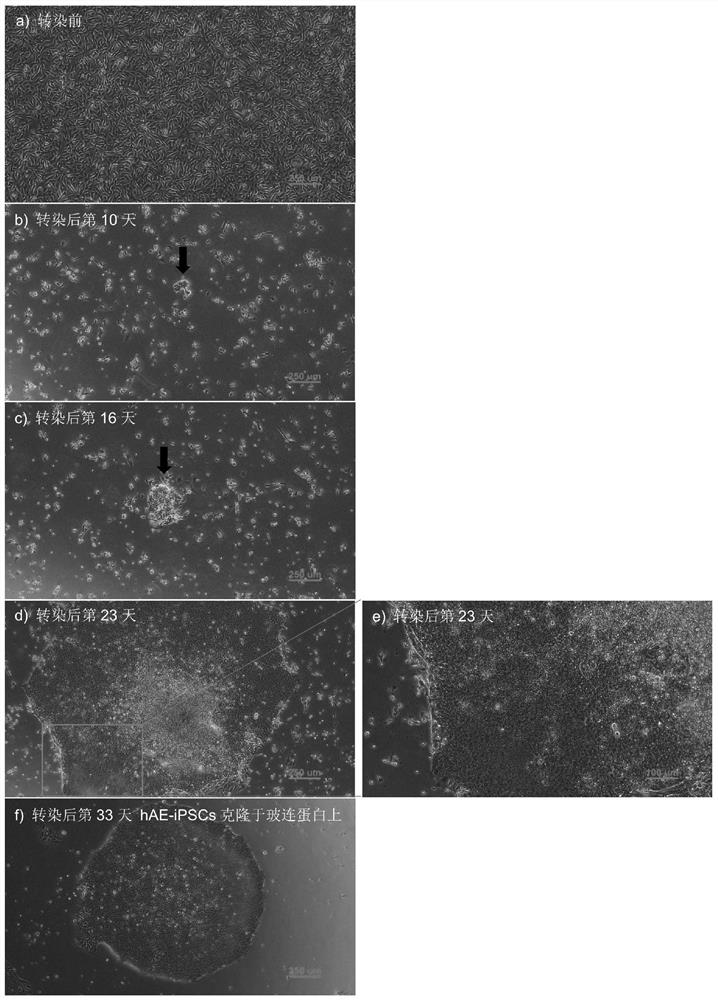
[0046] Example 1 Reprogramming steps and effects of human amniotic epithelial cells
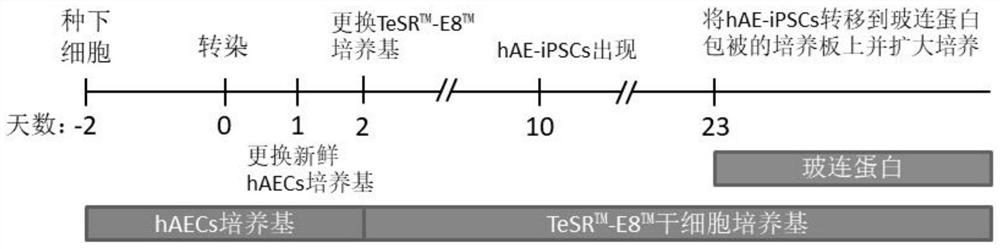
[0047] Steps of reprogramming human amniotic epithelial cells into hAE-iPSCs in the present invention ( figure 1 ) and the effects are as follows: Day -2: planting cells
[0048] Take 5×10 4 Cells per square centimeter The hAECs cultured in vitro within two passages were plated in a 6-well culture plate, that is, each well of the 6-well culture plate was planted with 4.8×10 5 hAECs.
[0049] Day 0: Transfection
[0050] Take one well of a 6-well culture plate as an example
[0051] 1) Two hours before transfection, replace the cells with fresh human amniotic epithelial cell medium hAECM;
[0052] 2) Dilute the plasmids: Take 1 μg each of the plasmids pCXLE-hOCT3 / 4-shp53-F, pCXLE-hSK, and pCXLE-hUL and mix them with 100 μL DMEM;
[0053] 3) Add 3 μL Neofect directly to the DNA diluent TM Gently mix the transfection reagent, let it stand at room temperature for 20 minutes, and the transf...
Embodiment 2
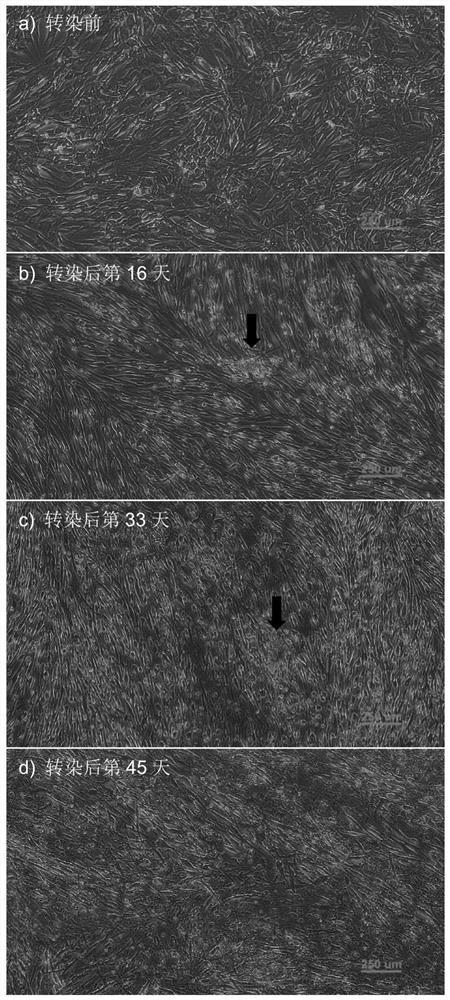
[0067] Embodiment 2 Reprogramming steps and effects of human dermal fibroblasts
[0068] The reprogramming steps and effects of the above method in human dermal fibroblasts are as follows:
[0069] Day -2: Seeding cells
[0070] Take 5×10 4 The density of cells per square centimeter spread the human dermal fibroblasts cultured in vitro within four generations in a 6-well culture plate, that is, each well of the 6-well culture plate was 4.8×10 5 Individual dermal fibroblasts.
[0071] Before transfection, it was observed that the morphology and viability of human dermal fibroblasts in the culture plate were normal ( image 3 a).
[0072] Day 0: Transfection
[0073] Take one well of a 6-well culture plate as an example
[0074] 1) Two hours before transfection, replace the cells with fresh human fibroblast culture medium;
[0075] 2) Dilute the plasmids: Take 1 μg each of the plasmids pCXLE-hOCT3 / 4-shp53-F, pCXLE-hSK, and pCXLE-hUL and mix them with 100 μL DMEM;
[0076...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com