Bone-marrow mesenchymal stem cell culture medium and application thereof
A bone marrow mesenchymal and cell culture technology, applied in the field of bone marrow mesenchymal stem cell culture medium, can solve the problems of bMSC in general proliferation ability in vitro, limitation of clinical cell therapy, low self-renewal ability, etc. The effect of decreased sex and large nucleoplasmic ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
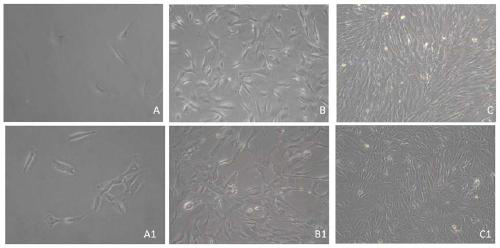
[0028] Example 1 Analysis of conventional culture medium and bone marrow mesenchymal stem cell culture medium of the present invention for bone marrow mesenchymal stem cell culture
[0029] The control group adopts conventional culture medium, and the components of conventional culture medium are: 10% fetal bovine serum (volume percentage), 1% penicillin-streptomycin double antibody (volume percentage), low-sugar DMEM; Experimental group adopts bone marrow mesenchyme of the present invention See Table 1 for details of the stem cell culture medium and the medium composition of the experimental group.
[0030] Table 1 The medium composition of the experimental group
[0031]
[0032]
[0033] The two media were used for primary culture and subculture respectively.
[0034] 1. Primary culture:
[0035] For the bone marrow derived from clinical patients, wash it twice with PBS, use 4°C pre-cooled 0.16M Tris-NH4Cl to break red for 5 minutes, centrifuge at 1000 rpm for 5 min...
Embodiment 2
[0040] Example 2 Detection of bMSC-specific markers by flow cytometry
[0041] Take the bMSC cells in the logarithmic growth phase of the experimental group and the control group in Example 1 respectively, wash the cells three times with PBS, digest the cells with 0.05% Trypsin, stop the digestion of the medium and blow until a single cell suspension is obtained; about 10 5 ~10 6 According to the antibody instructions, add the corresponding detection amount of fluorescently labeled antibodies (CD34-FITC, CD45-FITC, CD106-FITC, CD44-FITC, HLA-1-FITC; CD90-FITC, CD29-PE,) and the corresponding isotype For the control antibody, mix well and place at room temperature in the dark for 30 minutes. Add 2ml of PBS, centrifuge at 1000g for 5min, discard the supernatant, and add 300μl of PBS to resuspend the cells. For flow cytometry detection, cells were acquired with Cell quest software, and the percentage of positive cells was analyzed. See Table 2 for analytical results. Cell sur...
Embodiment 3
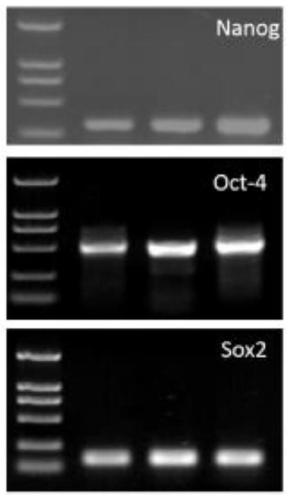
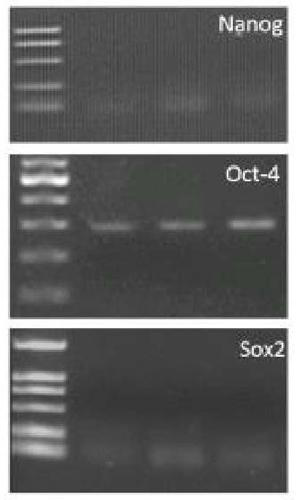
[0045] The pluripotency gene expression identification (RT-PCR detection) of embodiment 3bMSC
[0046] 1. Extraction of total cellular RNA: using the TRIzol Total RNA Extraction Kit (Shanghai Sangon Bioengineering Technology Service Co., Ltd.), respectively extract the bone marrow mesenchymal stem cells prepared in the experimental group and the control group in Example 1. For the total RNA at passage 7, the purity and quantity of the extracted RNA were measured by a spectrophotometer, and stored at -70°C.
[0047] 2. Synthesis of cDNA: 25 μl reaction system: 2 μg of cellular RNA sample, 3 μl of random primer or Oligo dT-Adaptor Primer, 10 μl of RNase Free ddH2O. 70°C, 10min. Quench on ice, 1 min. Centrifuge briefly. Add 1.5μl of 10mM dNTP, 0.5μl of RNase Inhibitor (25units), 1μl of M-MMLV Reverse Transcriptase (200units), 5μl of 5×RNA PCR Buffer, 4μl of RNase Free ddH2O, lightly flick, and put in a PCR machine at 37°C for 100min. After the reaction, mix well and store at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com