A kind of preparation method of clinical grade placental mesenchymal stem cells
A technology for stem cells and placenta, applied in the field of biomedicine, can solve the problems of unclear use for clinical-grade cell expansion and culture, complicated operation, and influence on cell adherent growth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
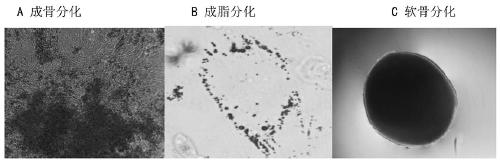
[0089] The invention provides a method for preparing clinical-grade placental mesenchymal stem cells, including the steps of cell separation, primary and passage serum-free expansion, and the like.
[0090] In the present invention, suitable materials include placental tissue materials, usually human placental amnion or chorion tissue.
[0091] In the method of the present invention, there is no particular limitation on the volume of the placental tissue material used as a raw material. Typically, the volume is 0.1-5ml, preferably 0.2-2ml, or by weight, 0.1-5g, preferably 0.2-2g.
[0092] The volume ratio of placental amnion or chorion tissue of the present invention to the combined digestive fluid of the present invention is not particularly limited, usually 1g:2-4ml (weight / volume ratio).
[0093] In the present invention, the digestion conditions with combined enzymes include: digestion at 37±2°C; and / or digestion for 6-24 hours, preferably 8-20 hours, more preferably 12-1...
Embodiment 1
[0138] Preparation of primary placental mesenchymal stem cells
[0139] 1. The placenta samples are qualified after strict infectious virus testing (hepatitis B virus, hepatitis C virus, HIV, syphilis and cytomegalovirus) and the results are negative, and enter the cell separation process.
[0140] 2. In a class 100 clean environment, such as an ultra-clean workbench, cut the placental amniotic membrane or chorionic tissue into 1% penicillin / streptomycin PBS solution, remove the remaining blood stains, cut it into pieces mechanically with scissors, and collect In a 15ml centrifuge tube, add 1:3-4ml volume ratio (80U / ml, 0.008wt% working concentration of collagenase type IV, trypsin) combined digestion solution; after digestion at 37°C for 12-18h, preferably 16h, fully blown Mix well, transfer the supernatant suspension to a new 15ml centrifuge tube, centrifuge at 300-500g for 5min, and harvest the cell pellet.
[0141] 3. Add 1-2ml red blood cell lysate to the cells harvested...
Embodiment 2
[0145] Serum-free subculture, harvest, and passaging of primary cells
[0146] Add the primary placental mesenchymal stem cells obtained in Example 1 to resuspend in serum-free medium, and press 1:3-6 (cell number dilution ratio) or 3000-6000 cells / cm 2 Density passage expansion.
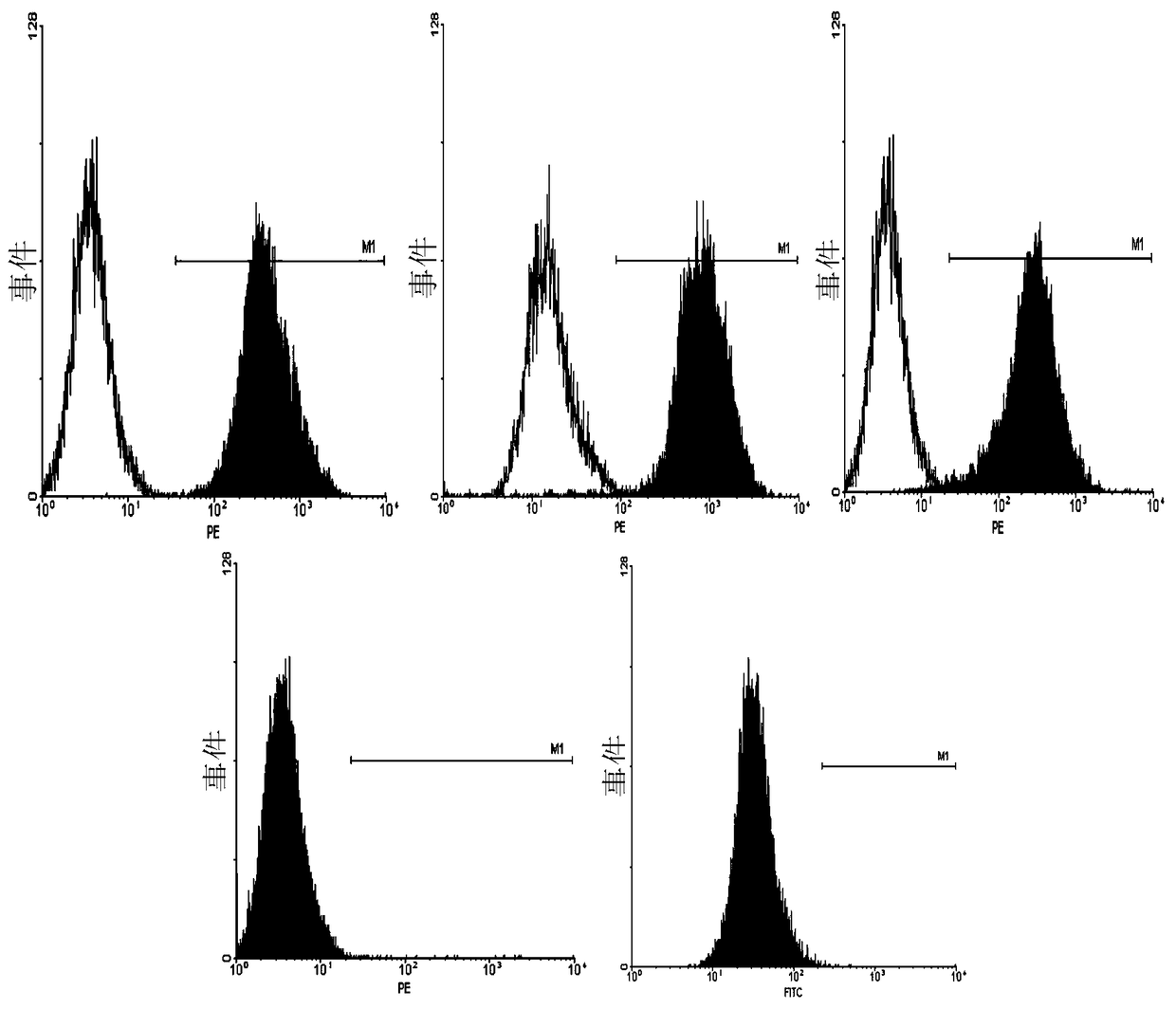
[0147] After 1-2 amplification passages, low-passage, 10 7 Cells of the above order of magnitude were stored at -196°C, and the cells were analyzed by flow cytometry for surface markers.
[0148] Results of cell morphology identification
[0149] Cell morphology was observed on the amplified cells, and the results were as follows:
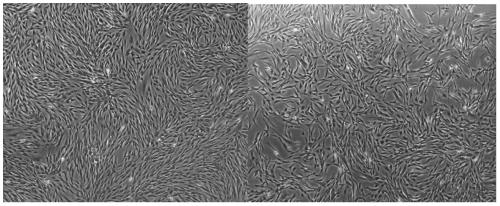
[0150] Placental mesenchymal stem cells obtained by the separation and serum-free expansion method. The morphology under the inverted microscope is as attached figure 1 As shown, the adherent cells of the P5 generation were spindle-shaped and polygonal, with uniform cell shape and size and strong cell proliferation ability.
[0151] Flow Cytometry Surface Marke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com