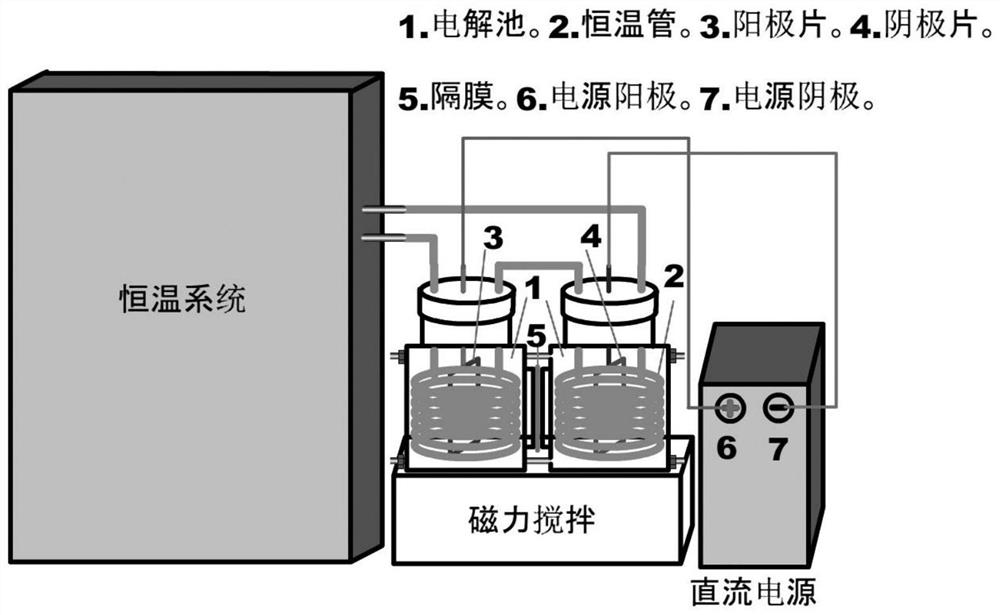
Method for preparing oxidized chitosan and derivatives thereof by electrolytic method
A technology for oxidizing chitosan and its derivatives, which is applied in the field of biomedical materials, can solve the problems of no oxidized chitosan and its derivatives, and achieve the effects of saving manufacturing costs, less dosage, and lower prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Add 200ml of dilute sulfuric acid solution with a concentration of 0.3mol / L in the anode tank of the electrolytic cell, weigh 0.25 grams of sodium iodate, add it to the anode tank, and stir to make it dissolve completely;
[0039] (2) Weigh 4 grams of chitosan, add it to the anode tank, and stir to dissolve it completely;
[0040] (3) Add 300ml of dilute sulfuric acid with a concentration of 0.15mol / L to the cathode tank of the electrolytic cell;
[0041] (4) Turn on the DC power supply, set the current to 1.0A, control the temperature at 50°C, and react for 3 hours in the dark;
[0042] (5) Add 200ml of absolute ethanol, collect the precipitate, and collect the waste liquid;
[0043] (6) Pour the precipitate into a dialysis bag with a molecular weight cut-off of 8000Da, place the dialysis bag in 500ml of distilled water, dialyze for 12 hours, and collect the distilled water after the first dialysis;
[0044] (7) Place the dialysis bag in 1500ml of distilled water, ...
Embodiment 2
[0051] (1) Add 100ml of dilute sulfuric acid solution with a concentration of 0.2mol / L in the anode tank of the electrolytic cell, weigh 0.5 grams of sodium iodate, add it to the anode tank, and stir to make it dissolve completely;
[0052] (2) Weigh 10 grams of chitosan oligosaccharide, add it to the anode tank, and stir to dissolve it completely;
[0053] (3) Add 250ml of dilute sulfuric acid with a concentration of 0.3mol / L to the cathode tank of the electrolytic cell;
[0054] (4) Turn on the DC power supply, set the current to 1.5A, control the temperature at 16°C, and react for 2 hours in the dark;
[0055] (5) Pour the reactant into a dialysis bag with a molecular weight cut-off of 500Da, place the dialysis bag in 1500ml of distilled water, change the distilled water every 12 hours, and dialyze for 24 hours;
[0056] (6) Take out the product in the dialysis bag, and use freeze-drying to obtain oxidized chitosan oligosaccharide;
[0057] In this example, the platinum s...
Embodiment 3
[0059] (1) Add 300ml of dilute sulfuric acid solution with a concentration of 0.15mol / L to the anode tank of the electrolytic cell, weigh 1 g of sodium iodate, add it to the anode tank, and stir to dissolve it completely;
[0060] (2) Weigh 10 grams of chitosan diquaternary ammonium salt, add it to the anode tank, and stir to dissolve it completely;
[0061] (3) Add 200ml of dilute sulfuric acid with a concentration of 0.2mol / L to the cathode tank of the electrolytic cell;
[0062] (4) Turn on the DC power supply, set the current to 0.6A, control the temperature at 5°C, and react for 5.0 hours under dark conditions;
[0063] (5) Pour the reactants into a dialysis bag with a molecular weight cut-off of 3000Da, place the dialysis bag in 500ml of distilled water for 12 hours, and collect the dialyzed distilled water;
[0064] (6) Place the dialysis bag in 2000ml of distilled water for 12 hours and change the distilled water every 12 hours for 48 hours;
[0065] (7) Take out the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com