Synthetic method of fullerene spiro derivative
A synthesis method, fullerene technology, applied in the direction of organic chemistry, can solve the problems of limited preparation methods, less fullerene and spiro ring structure, etc., and achieve the effects of environmental friendliness, wide application range of substrates, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
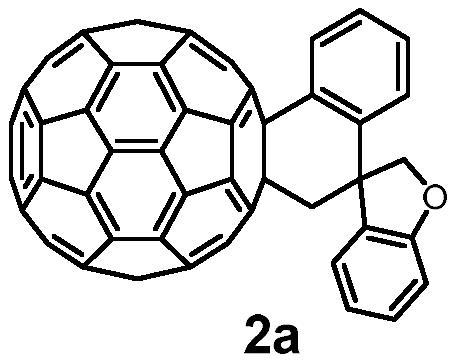
[0014] Preparation of Fullerenospiro Derivative 2a
[0015]
[0016] Reaction steps:
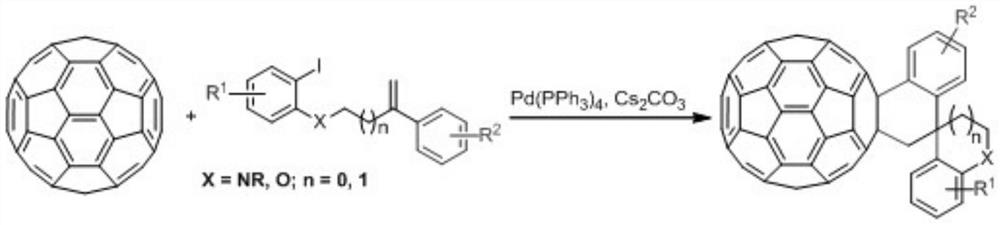
[0017] Take a dry 15mL Shrek tube, add fullerene C to it sequentially 60 (36.0mg, 0.05mmol), compound (0.1mmol), Pd(PPh 3 ) 4 (2.9mg, 0.0025mmol) and Cs 2 CO 3 (32.6mg, 0.1mmol), ultrasonically dissolved the solid in anhydrous CB (6mL), placed the reaction tube in an oil bath at 130°C for 24 hours, lifted the reaction tube to cool to room temperature after the reaction, and evaporated the solvent in vacuo The residue was separated on a silica gel column with CS 2 as eluent to recover unreacted fullerene C 60 , then use the volume ratio CS 2 / DCM=8:1 was used as the eluent to obtain the target product fullerene and spiro derivative 2a, and the isolated yield of product 2a was 48%.
[0018] Product 2a: 1 H NMR (400MHz, CDCl 3 / CS 2 )δ7.77(d, J=7.6Hz, 1H), 7.69(d, J=7.2Hz, 1H), 7.57–7.49(m, 2H), 7.37(d, J=7.6Hz, 1H), 7.23( t,J=8.0Hz,1H),6.91(t,J=8.0Hz,2H),6.63(d,J=9.2Hz,1H),5.0...
Embodiment 2
[0020] Preparation of fullerene and spiro derivative 2b
[0021]
[0022] Reaction steps:
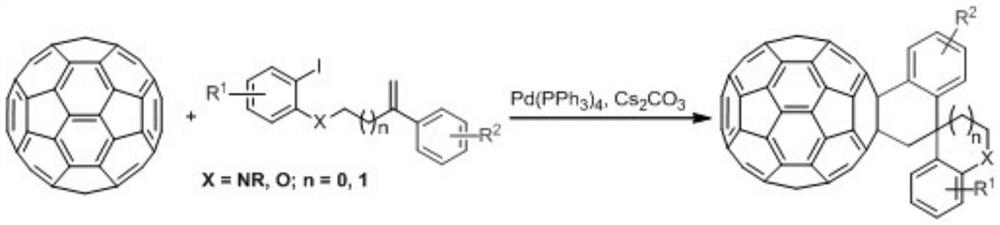
[0023] Take a dry 15mL Shrek tube, add fullerene C to it sequentially 60 (36.0mg, 0.05mmol), compound (0.1mmol), Pd(PPh 3 ) 4 (2.9mg, 0.0025mmol) and Cs 2 CO 3 (32.6mg, 0.1mmol), ultrasonically dissolved the solid in anhydrous CB (6mL), placed the reaction tube in an oil bath at 130°C for 24 hours, lifted the reaction tube to cool to room temperature after the reaction, and evaporated the solvent in vacuo The residue was separated on a silica gel column with CS 2 as eluent to recover unreacted fullerene C 60 , then use the volume ratio CS 2 / DCM=8:1 was used as the eluent to obtain the target product fullerene and spiro derivative 2b, and the isolated yield of product 2b was 40%.
[0024] Product 2b: 1 H NMR (600MHz, CDCl 3 / CS 2 )δ7.83(d, J=7.8Hz, 1H), 7.69(d, J=7.2Hz, 1H), 7.56(t, J=7.8Hz, 1H), 7.53(t, J=7.8Hz, 1H) ,6.92(s,1H),6.81–6.78(m,2H),6.60(d,J=9.0Hz,1H),5.01(d...
Embodiment 3
[0026] Preparation of fullerene and spiro derivative 2c
[0027]
[0028] Reaction steps:
[0029] Take a dry 15mL Shrek tube, add fullerene C to it sequentially 60 (36.0mg, 0.05mmol), compound (0.1mmol), Pd(PPh 3 ) 4 (2.9mg, 0.0025mmol) and Cs 2 CO 3 (32.6mg, 0.1mmol), ultrasonically dissolved the solid in anhydrous CB (6mL), placed the reaction tube in an oil bath at 130°C for 24 hours, lifted the reaction tube to cool to room temperature after the reaction, and evaporated the solvent in vacuo The residue was separated on a silica gel column with CS 2 as eluent to recover unreacted fullerene C 60 , then use the volume ratio CS 2 / DCM=8:1 was used as the eluent to obtain the target product fullerene and spiro derivative 2c, and the isolated yield of product 2c was 45%.
[0030] Product 2c: 1 H NMR (600MHz, CDCl 3 / CS 2 )δ7.72(d, J=7.2Hz, 1H), 7.68(d, J=6.6Hz, 1H), 7.58–7.53(m, 2H), 7.44(s, 1H), 7.31(d, J=8.4 Hz,1H),6.78(d,J=8.4Hz,1H),6.63(d,J=9.6Hz,1H),5.04(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com