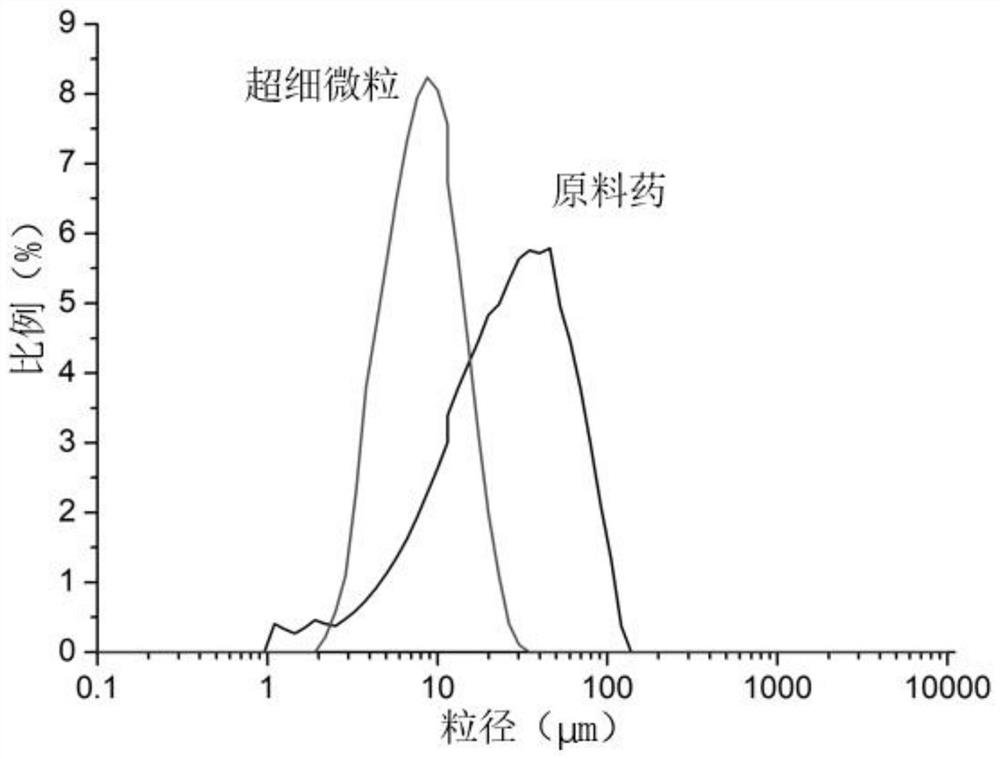
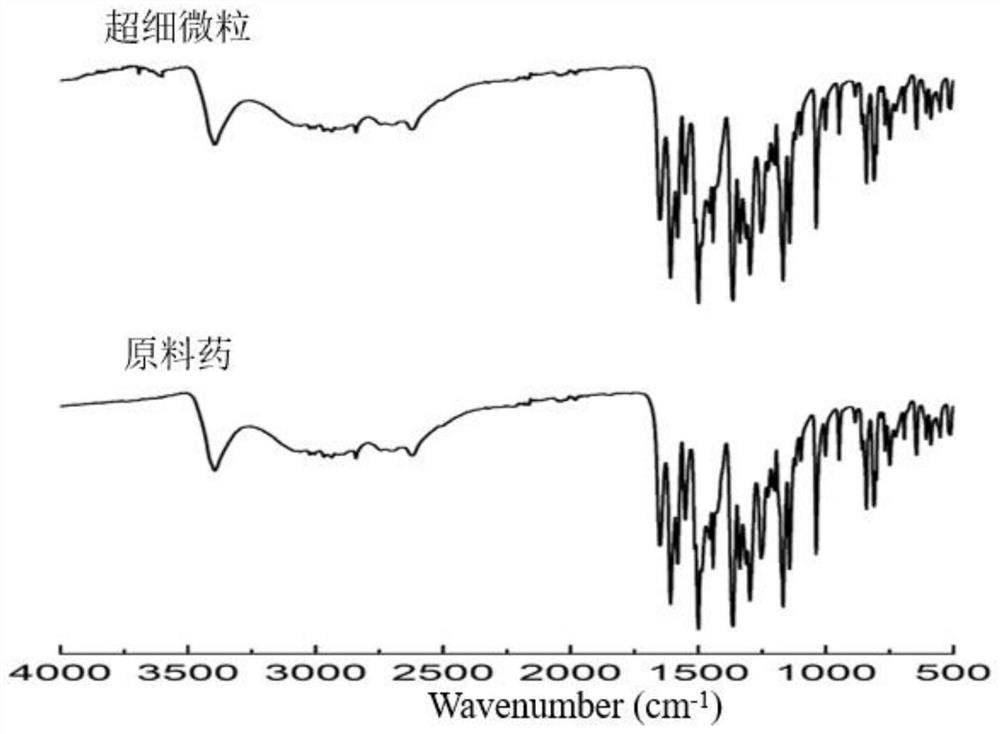
Method for preparing diosmetin derivative ultrafine particles
A technology of diosmin and ultrafine particles, which is applied in educts, bulk chemical production, organic chemistry, etc., can solve the problems of limited development and promotion, small compound range, etc., and achieve improved dissolution performance, uniform distribution, and particle size small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Single factor investigation of the influence of various factors on the particle size and recovery rate of diosmin derivative ultrafine particles
[0039] Single factor experiment: Effect of solvent type on particle size of ultrafine particles of diosmin derivatives
[0040] Ethanol and ethanol-DMSO with a volume ratio of 50:1, 20:1, and 10:1 were used as solvents for the derivatives, and other parameters were crystallization pressure 11MPa, crystallization temperature 35°C, solution mass concentration 9mg / mL, solution volume Flow 1.0mL / min, CO 2 The flow rate is 3.0-3.5L / min. The diosmin derivative 1 could not obtain shaped ultrafine particles under the above solvent conditions, and the diosmin derivative 2 could obtain shaped ultrafine particles when the solvent was ethanol-DMSO at a volume ratio of 20:1.
[0041] Therefore, other parameters were optimized with the goal of diosmin derivative 2, and ethanol-DMSO with a volume ratio of 20:1 was selected as t...
Embodiment 2
[0052] Example 2: Preparation of diosmin derivative 2 ultrafine particles using optimal process conditions
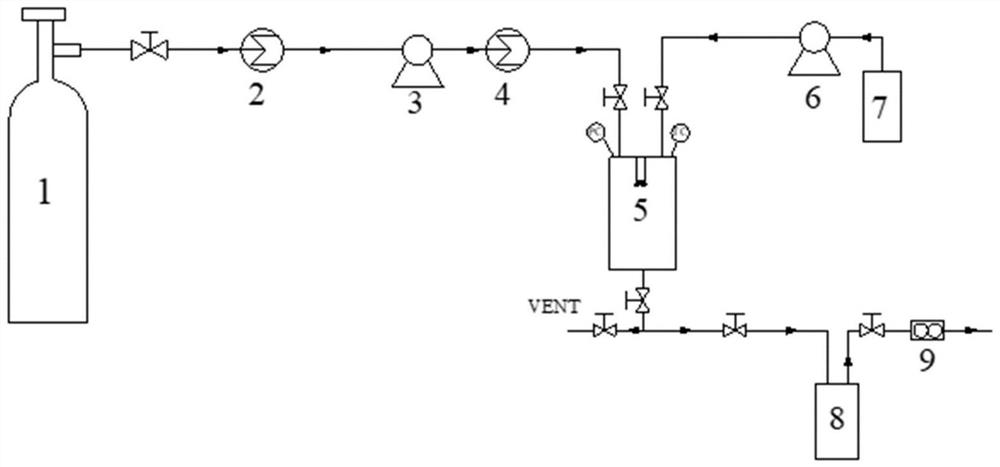
[0053] The application of supercritical fluid enhanced solution dispersion technology to prepare diosmin derivative 2 ultrafine particles comprises the following steps:
[0054] Step S1, dissolving diosmin derivative 2 in an organic solvent to obtain a compound solution;
[0055] Step S2, adjust the temperature of the crystallization tank to the experimental value, and then add CO 2 Pass into the crystallization kettle, and pressurize to set experimental value;
[0056] Step S3, continue to feed CO 2 , maintaining the temperature and pressure in the crystallization tank constant, while passing the compound solution prepared in step S1 into the crystallization tank;
[0057] Step S4, after the compound solution is passed through, continue to pass through CO 2 65min, while adjusting the CO through the rotameter 2 The flow rate is maintained within a certain flow rate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com