A kind of C2-sulfonamidoindole derivative and its preparation method
A technology of sulfonylaminoindole derivatives and sulfonyl azide, which is applied in the new synthesis field of indole derivatives, can solve the problems of excessive catalyst amount and low yield, and achieve simple and easy operation process and simple steps , The effect of reducing the reaction material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation steps of C2-sulfonamidoindole derivatives are as follows:
[0040]
[0041] Add 1-(3-phenyl-1-(pyridin-2-yl)-1H-indol-2-yl)butan-1-ol (68 mg, 0.2 mmol), benzenesulfonyl azide (73 mg, 0.4mmol), [Cp * Rh(CH 3 EN) 3 ][SbF 6 ] 2 (10mg, 0.01mmol) and 1ml of anhydrous 1,2-dichloroethane, stirred and reacted at 85°C for 48h, stopped heating, cooled to room temperature, spin-dried, and further separated and purified by column chromatography to obtain 63mg of the product , Yield: 74%.
[0042] The structural characterization data of the product obtained in this embodiment are as follows:
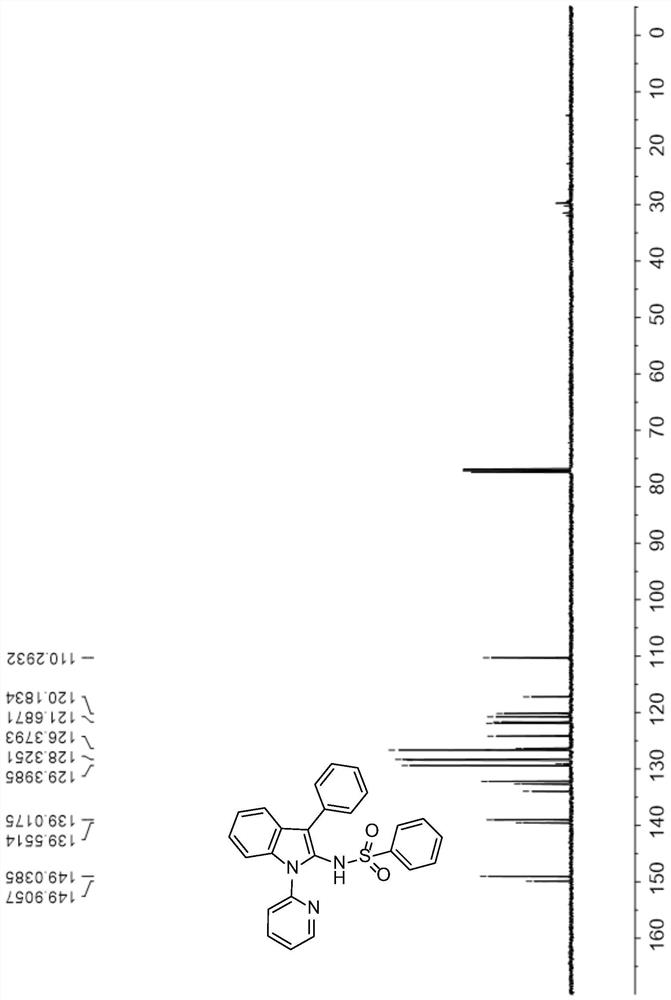
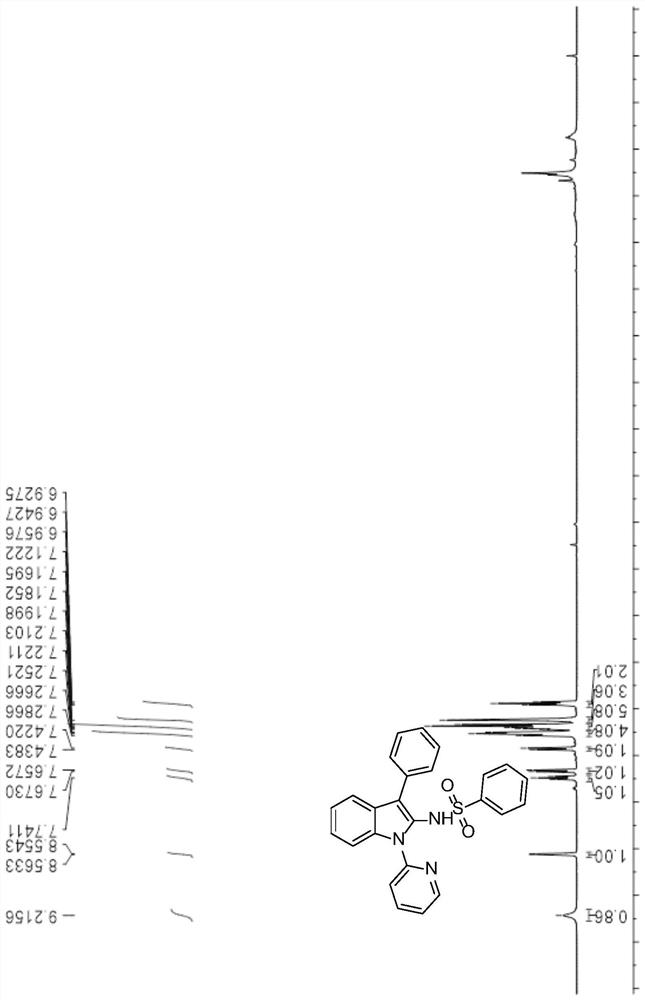
[0043] 1 H NMR (500MHz, CDCl 3 )δ9.22(s,0.86H),8.56(d,J=4.5Hz,1H),7.74(t,J=7.7Hz,1H),7.67(d,J=7.9Hz,1H),7.43(d , J=8.2Hz, 1H), 7.30–7.24(m, 4H), 7.22–7.16(m, 5H), 7.12(s, 3H), 6.94(t, J=7.5Hz, 2H). figure 1 .
[0044] 13 C NMR (126MHz, CDCl 3 )δ149.9, 149.0, 139.5, 139.0, 133.9, 132.6, 132.2, 129.4, 129.0, 128.4, 128.3, 126.6, 126.6, 126.3, 124.1, 121.9, 121.6, ...
Embodiment 2
[0048] The preparation steps of C2-sulfonamidoindole derivatives are as follows:
[0049]
[0050] In a sealed tube add (3-phenyl-1-(pyrimidin-2-yl)-1H-indol-2-yl)methanol (69 mg, 0.2 mmol), p-toluenesulfonyl azide (80 mg, 0.4 mmol) , [Cp * Rh(CH 3 EN) 3 ][SbF 6 ] 2 (10mg, 0.01mmol) and 1ml of anhydrous 1,2-dichloroethane, stirred and reacted at 85°C for 48h, then stopped heating, cooled to room temperature, spin-dried, and further separated and purified by column chromatography to obtain 24mg of the product. Yield: 14%.
[0051] The structural characterization data of the product obtained in this embodiment are as follows:
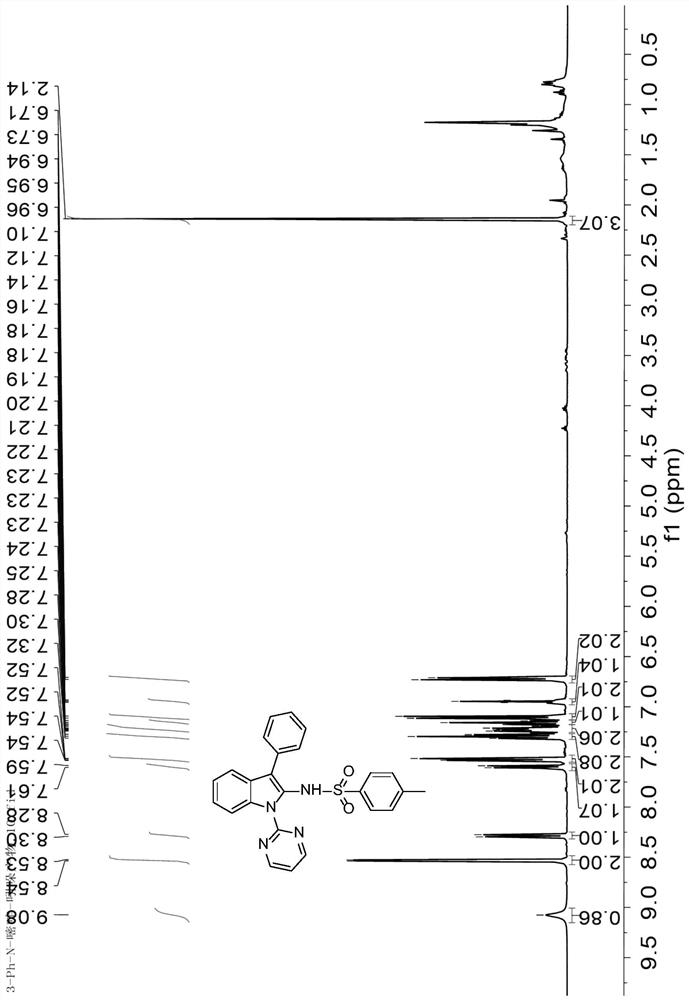
[0052] 1 H NMR (400MHz, Chloroform-d) δ9.08(s, 0.86H), 8.53(d, J=4.8Hz, 2H), 8.29(d, J=8.3Hz, 1H), 7.60(d, J=7.9 Hz, 1H), 7.55–7.48(m, 2H), 7.30(t, J=7.5Hz, 2H), 7.26–7.18(m, 2H), 7.15(d, J=7.4Hz, 1H), 7.11(d , J=8.0Hz, 2H), 6.95(t, J=4.8Hz, 1H), 6.72(d, J=8.0Hz, 2H), 2.14(s, 3H). image 3 .
[0053] 13 C NMR (101MHz, CDCl 3 )δ157.8, 157.3...
Embodiment 3
[0058] The preparation steps of C2-sulfonamidoindole derivatives are as follows:
[0059]
[0060] Add 1-(3-phenyl-1-(pyridin-2-yl)-1H-indol-2-yl)butan-1-ol (68 mg, 0.2 mmol), thiophenesulfonyl azide (80 mg, 0.4mmol), [Cp * Rh(CH 3 EN) 3 ][SbF 6 ] 2 (10mg, 0.01mmol) and 1ml of anhydrous 1,2-dichloroethane, stirred and reacted at 85°C for 48h, then stopped heating, cooled to room temperature, spin-dried, and further separated and purified by column chromatography to obtain 72mg of the product. Yield: 84%.
[0061] The structural characterization data of the product obtained in this embodiment are as follows:
[0062] 1 H NMR (400MHz, Chloroform-d) δ9.17(s,0.91H),8.62(m,1H),7.84(m,1H),7.78(m,1H),7.55–7.49(m,3H),7.38 –7.31 (m, 4H), 7.30 – 7.21 (m, 4H), 6.91 (dd, J=3.8, 1.4Hz, 1H), 6.62 (dd, J=5.0, 3.8Hz, 1H). Figure 5 .
[0063] 13 C NMR (101MHz, CDCl 3 )δ150.0, 149.1, 140.0, 138.9, 133.8, 132.8, 132.4, 132.2, 129.5, 128.4, 127.1, 126.8, 126.7, 126.1, 124.3, 122.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com