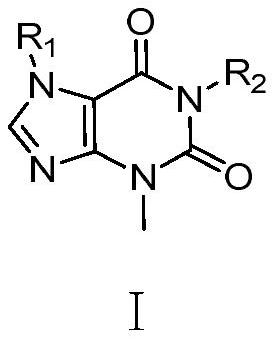
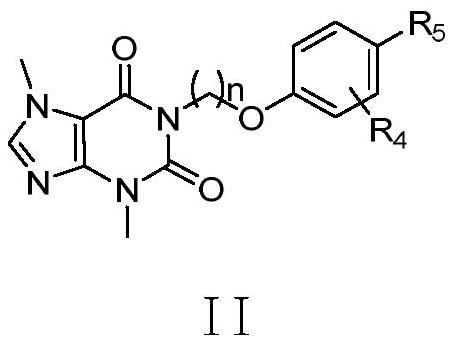
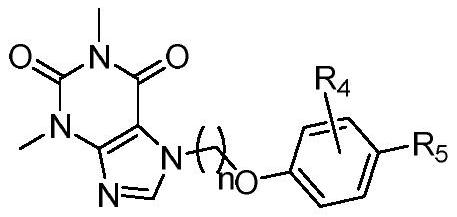
Xanthine aromatic acid ether derivatives and application thereof
A xanthine aromatic acid ether and derivative technology, applied in the field of chemistry, can solve the problem of not providing satisfactory therapeutic effects, and achieve the effects of excellent neuroprotective activity, platelet aggregation inhibition, and significant neuroprotective function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Compound 4-(2-(3,7-Dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-1-yl)ethoxy)benzoic acid (F -1) synthesis:
[0033]
[0034] Step 1 Add compound 1 (10.0mmol, 1.0eq) and DMSO (30mL) to a 100mL dry round bottom flask, add 60% NaH (15.0mmol, 1.5eq) in batches under stirring at room temperature, and continue stirring for 10min, The compound 1,2-dibromoethane (40.1 mmol, 4.0 eq) was added to the reaction solution, stirred at 60° C. for 6 h, TLC showed that the reaction was almost complete. The reaction solution was diluted with 300 mL of water, extracted with dichloromethane (80 mL×3), washed 3 times with saturated brine, dried over anhydrous sodium sulfate, filtered with suction and concentrated the filtrate under reduced pressure, and the residue was separated by silica gel column to obtain intermediate 2 , as a white solid, yield 45%.
[0035] Step 2 Add intermediate 2 (2.3 mmol, 1.0eq) successively in a 250mL dry round bottom flask, K 2 CO 3 (5.6mmol, 2.4eq) and...
Embodiment 2
[0038] Compound 4-(3-(3,7-Dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-1-yl)propoxy)benzoic acid (F -2) Synthesis:
[0039] Referring to the synthesis method of Example 1, the raw material of 1,2-dibromoethane was replaced by the raw material of 1,3-dibromopropane to obtain compound F-2 as a white solid with a yield of 74.8%. ESI-MS[M-H] - : m / z=357.03, 1 H NMR (400MHz, DMSO-d 6 )δppm: 8.01 (s, 1H, N = CH), 7.85 (d, J = 8.8Hz, 2H, ArH), 6.89 (d, J = 8.8 Hz, 2H, ArH), 4.12-4.04 (m, 4H, CH 2 CH 2 CH 2 ),3.84(s,3H,NCH 3 ), 3.39(s,3H,NCH 3 ),2.07-2.00(m,2H,CH 2 CH 2 CH 2 ).
Embodiment 3
[0041] Compound (E)-3-(4-(2-(3,7-Dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-1-yl)B Synthesis of oxy)-3-methoxyphenyl)acrylic acid (F-3):
[0042] Referring to the synthesis method of Example 1, the raw material of methyl p-hydroxybenzoate was replaced with the raw material of methyl ferulic acid to obtain compound F-3 as a white solid with a yield of 79.2%. ESI-MS[M-H] - : m / z=399.02, 1 H NMR (400MHz, DMSO-d 6 )δppm: 8.00 (s, 1H, N = CH), 7.44 (d, J = 16.0Hz, 1H, ArCH =), 7.21 (s, 1H, ArH), 7.13 (d, J = 8.3Hz, 1H, ArH ), 6.92(d, J=8.3Hz, 1H, ArH), 6.44(d, J=16.0Hz, 1H,=CH), 4.30(t, J=5.5Hz, 2H, OCH 2 ), 4.21(t, J=5.5Hz, 2H, NCH 2 ),3.84(s,3H,OCH 3 ),3.67 (s,3H,NCH 3 ),3.38(s,3H,NCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com