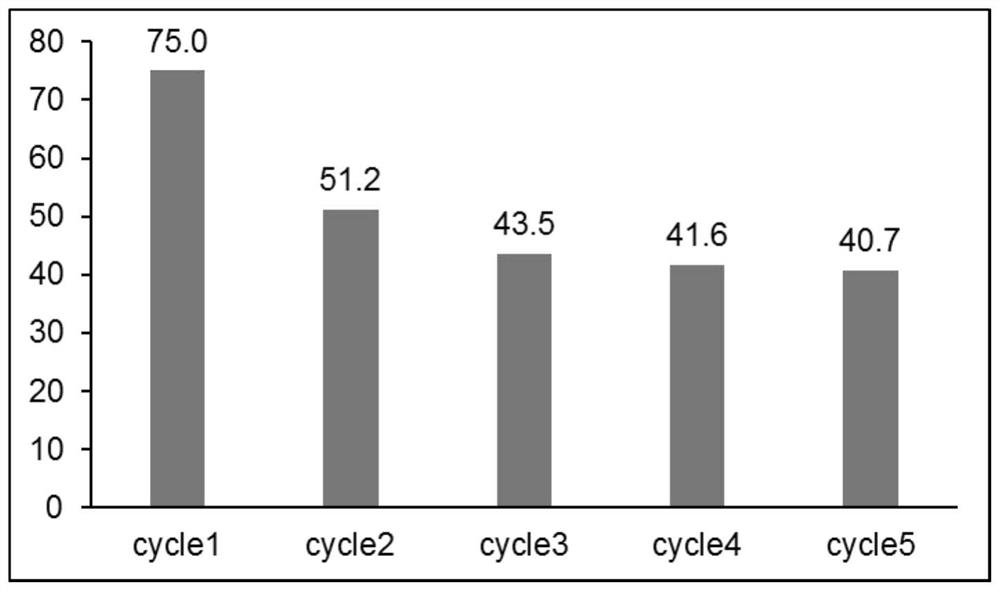
Kaolin-loaded nano-iron catalyst and application thereof in catalyzing cross dehydrogenation coupling reaction
A technology of cross dehydrogenation coupling and kaolin, which is applied in the direction of catalytic reaction, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., can solve the problems of difficult separation, recycling and reuse of catalysts, environmental pollution, high catalytic cost, etc., to achieve The effect of good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] In the present invention, the preparation method of the silane-modified kaolin preferably comprises the following steps:
[0028] The raw kaolin clay, n-heptane and silane coupling agent are mixed, stirred, left standing, solid-liquid separation, solid washed and dried in sequence to obtain silane modified kaolin.
[0029] In the present invention, the silane coupling agent is preferably 3-aminopropyltriethoxysilane and / or phenyltrimethoxysilane; when the silane coupling agent is 3-aminopropyltriethoxy During silane, the gained silane-modified kaolin is aminosilane-modified kaolin; when the silane coupling agent is phenyltrimethoxysilane, the silane-modified kaolin is phenylsilane-modified kaolin; when the silane coupling agent When the coupling agent is 3-aminopropyltriethoxysilane and phenyltrimethoxysilane, the obtained silane-modified kaolin is aminophenylsilane-modified kaolin.
[0030] In the present invention, the ratio of the raw kaolin, n-heptane and silane co...
Embodiment 1
[0057] Weigh 1g of kaolin into a beaker, add 30mL of n-heptane, stir evenly, add 1.2mmol of 3-aminopropyltriethoxysilane and 1.2mmol of phenyltrimethoxysilane, and stir at room temperature for 6h. After stirring, let stand for 12 hours, then wash with n-heptane, and dry for 8 hours to obtain the amino- and phenyl-modified kaolin carrier, denoted as NH 2 & Ph @kaolin.
[0058] The resulting NH 2 &Ph@kaolin at a concentration of 0.01mol / L FeCl 3 Immerse in the solution for 10h to remove the NH after immersion 2 & Ph @kaolin, utilizing NaBH 4 Reducted at room temperature for 10 minutes to obtain a kaolin-supported nano-iron catalyst with an iron loading of 3 wt%, denoted as 3% Fe / NH 2 & Ph @kaolin.
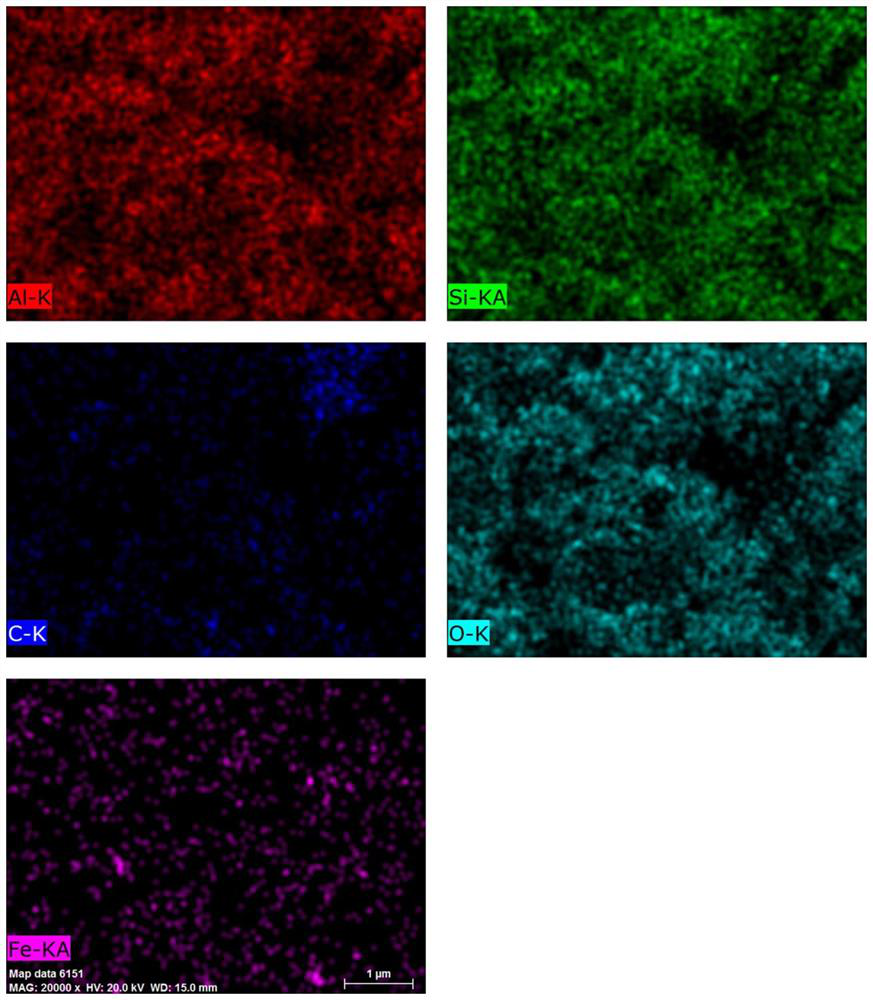
[0059] The resulting 3% Fe / NH 2 &Ph@kaolin's SEM element distribution map is as follows figure 1 shown. From figure 1 It can be seen that the nano-iron is uniformly distributed in the NH 2 &Ph@kaolin in the carrier.
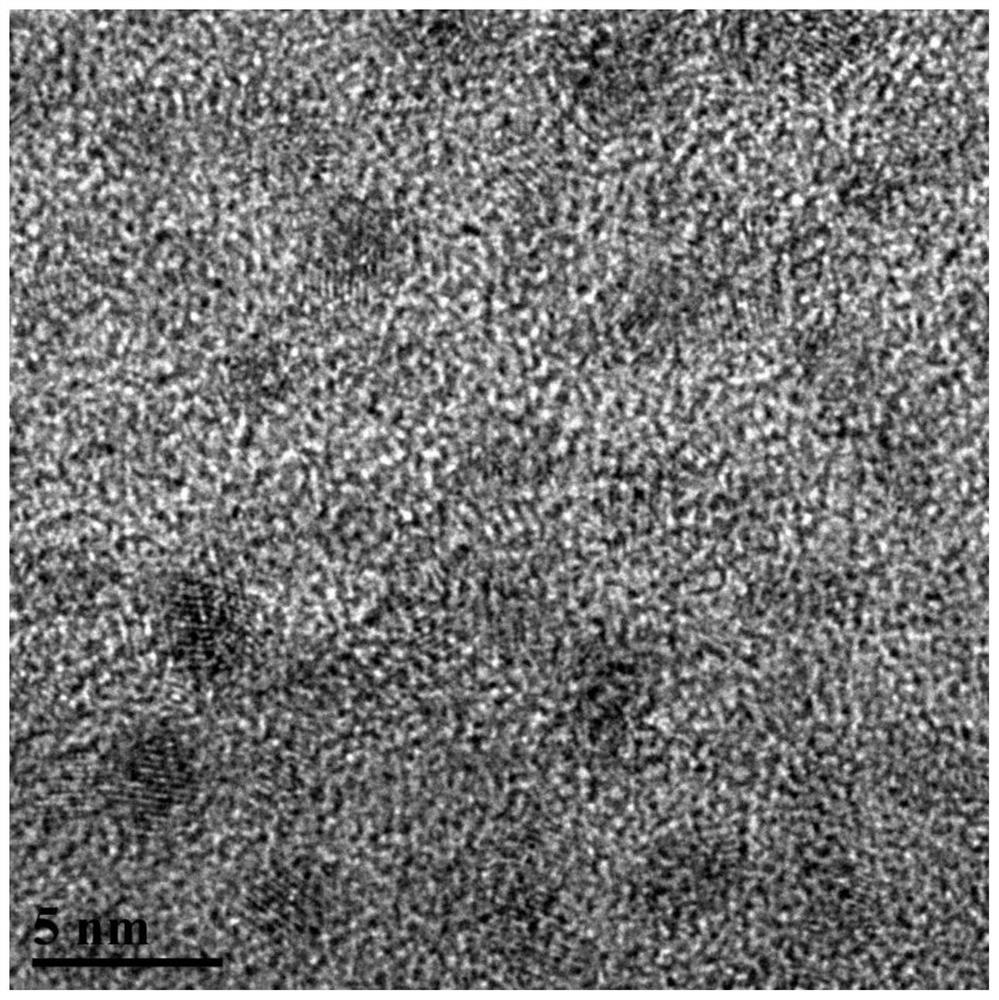
[0060] The resulting 3% Fe / NH 2 TEM image of &Ph@kaoli...
Embodiment 2
[0062] Weigh 1g of kaolin into a beaker, add 30mL of n-heptane, stir evenly, add 1.2mmol of 3-aminopropyltriethoxysilane, and stir at room temperature for 6h. After stirring, let stand for 12 hours, then wash with n-heptane, and dry for 8 hours to obtain a kaolin carrier modified with amino groups, which is denoted as NH 2 @kaolin.
[0063] The resulting NH 2 @kaolin FeCl at a concentration of 0.01mol / L 3 Immerse in the solution for 10h, take out the impregnated NH 2 @kaolin, using NaBH 4 Reducted at room temperature for 10 minutes to obtain a kaolin-supported nano-iron catalyst with an iron loading of 3 wt%, denoted as 3% Fe / NH 2 @kaolin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com