Perylene bisimide derivative and application thereof
A technology of perylene imide and derivatives, which is applied in the field of perylene imide derivatives and its application, and can solve the problems of perylene imide derivatives to be studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation and characterization of embodiment 1 perylene imide derivatives
[0020] PDIC-NC preparation and characterization. Dissolve N,N'-dimethylethylenediamine (2.5 mmol) in N-methylpyrrolidone (50 mL), then add 1,6,7,12-tetrachloro-3,4,9,10-perylenetetra Carboxylic acid dianhydride (1 mmol), under argon protection, react at 80 °C for 24 h, cool to room temperature, add 100 mL of acetone, stir at room temperature for 2 h, filter with suction, wash with water until neutral, add 2 M HCl at room temperature (20 mL ) reacted for 24 h, cooled and stood still, suction filtered, dried, dissolved in water and dialyzed for 24 h (dialysis bag specification: 500-1000Da), and the dialysate was freeze-dried (-62 °C, 24 h) to obtain PDIC-NC. 1 H NMR (CF 3 COOD, 400 MHz) δ 3.29 (d, J = 6.2 Hz, 12 H), 3.87 (s, 4 H), 4.88 (t, J = 5.0 Hz, 13 C NMR (100 MHz, CF 3 COOD): δ 167.32, 138.74, 136.30, 133.28,132.43, 125.30, 123.84, 123.83, 59.78, 46.10, 38.35; HRMS (MALDI-TOF): m / z:[M...
Embodiment 2
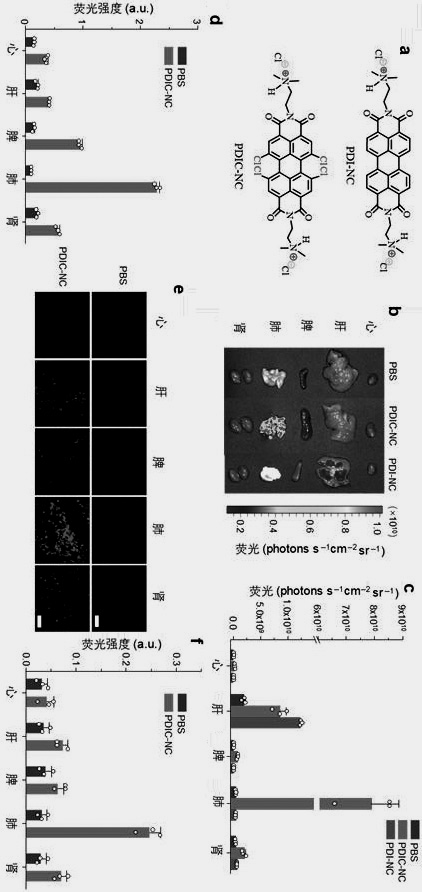
[0023] Embodiment 2 organ distribution of perylene imide derivatives
[0024] Normal mice were divided into groups (n = 3), and the above-mentioned peryleneimide derivatives PDI-NC and PDIC-NC aqueous solution were injected through the tail vein, and the main organs (heart, liver, spleen, lung, kidney) were collected by:
[0025] (1) Organ fluorescence imaging detection. In vivo imaging fluorescence imaging system was used to collect fluorescence images of major organs, quantify the fluorescence intensity, and analyze the distribution of compounds in major organs (n = 3);
[0026] (2) Quantitative analysis of organ fluorescence intensity. Take 50-70 mg of heart, liver, spleen, lung, and kidney, add tissue lysate, extract tissue fluid, carry out fluorescence spectrum detection, and quantitatively analyze the distribution of compounds in heart, liver, spleen, lung, and kidney according to the compound standard curve (n = 3);
[0027] (3) Organ tissue frozen section detectio...
Embodiment 3
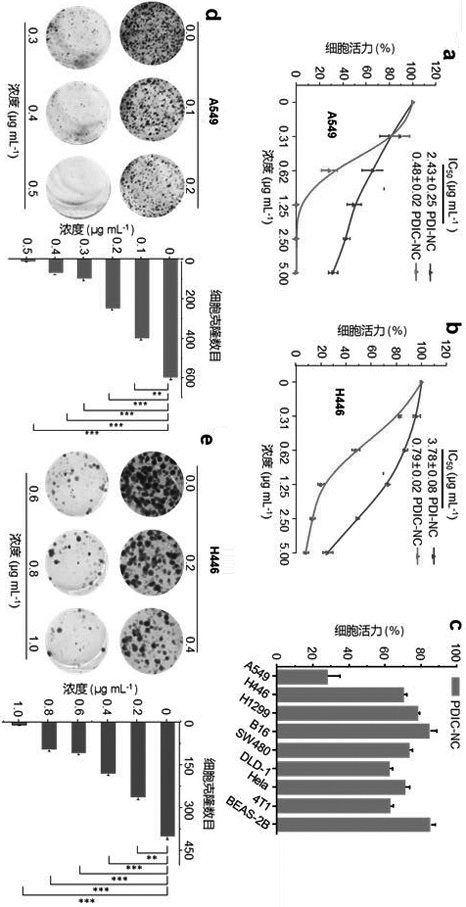
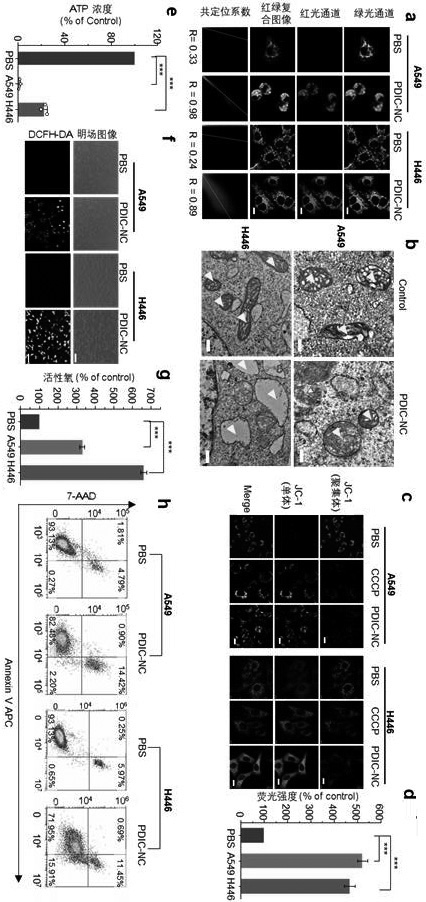
[0029] Example 3 The ability of peryleneimide derivatives to inhibit the proliferation of lung cancer cells
[0030] Non-small cell lung adenocarcinoma cell A549 and small cell lung cancer cell H446 were used as tumor cell models, and normal bronchial epithelial cells BESA-2B and human normal lung fibroblast HFL-1 were selected as cell comparison models. All cells were purchased from the ATCC cell bank in the United States. After the cells reached about 80% confluence, they were digested and subcultured with 0.25% trypsin, and the cells in the logarithmic growth phase were used for in vitro cell viability experiments.
[0031] (1) CCK-8 assay to determine the ability of peryleneimide derivatives to inhibit tumor cell proliferation
[0032] Cells were digested and inoculated in 96-well plates, each well containing 100 μL cell suspension (cell concentration per mL 5×10 4 ), placed in a cell culture incubator (5% CO 2 , 37°C) and cultured for 12 h, the blank control group, PBS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com