Method for detecting pentacyclic triterpenoids using lc-ms APCI source
A technology for pentacyclic triterpenes and pentacyclic triterpenes is applied in the field of determination of pentacyclic triterpenes, which can solve problems such as many fragments, and achieve the effects of high sensitivity, simple pretreatment, and elimination of false positive interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] 1. Sample pretreatment:
[0036] 1) Weigh 50±2.5mg of fresh sample, add 200μL extractant (methanol:MTBE=1:3), vortex for 5min, centrifuge at 12000r / min for 10min, after centrifugation, draw 100uL of the supernatant to a numbered glass liner Store in a sample vial at -20°C until LC-MS / MS analysis.
[0037] 2) Instruments and equipment
[0038]
[0039]
[0040] 2. Standard product processing
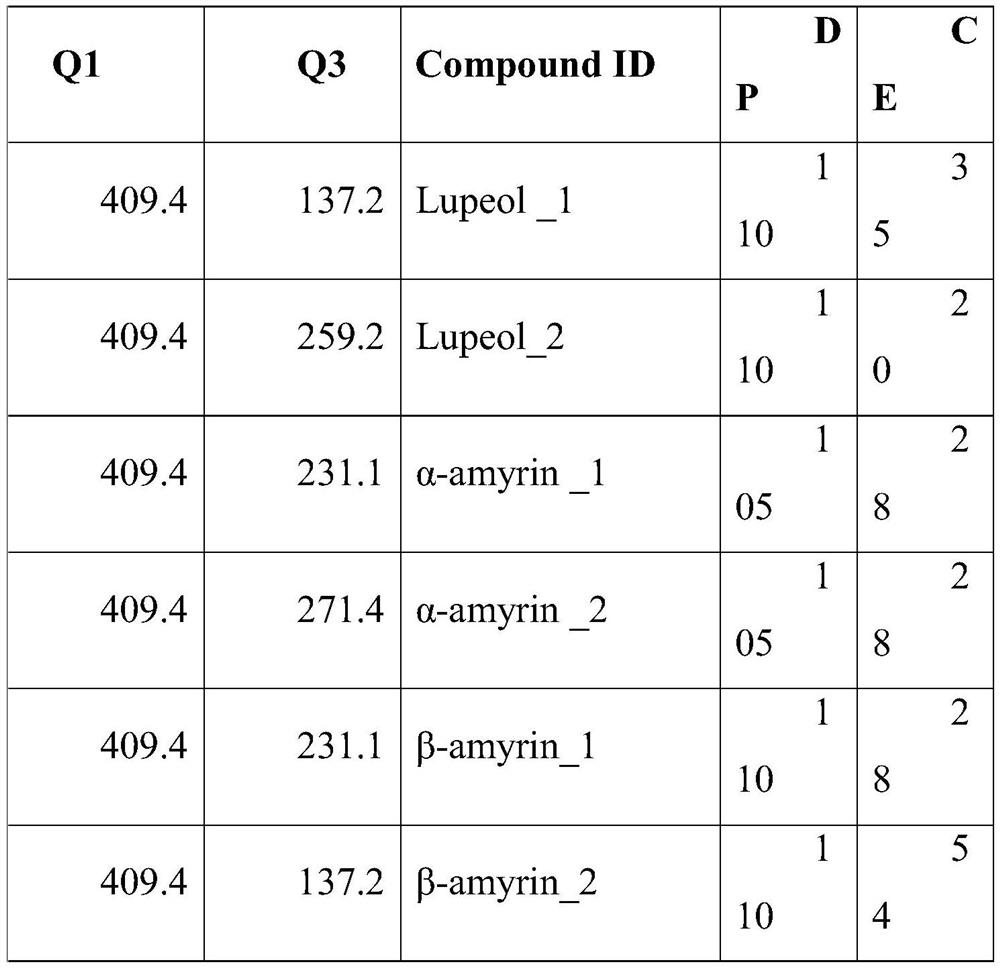
[0041] Dilute the purchased 3 kinds of pentacyclic triterpene standard products with MTBE / methanol (3:1) mixed solution for on-board detection to determine the ion pair information: prepare a 1ug / ml pentacyclic triterpene single standard for needle pump detection, and determine the adjustment parameters Precursor ions, product ions and optimized DP and CE, the specific information is as follows:
[0042]
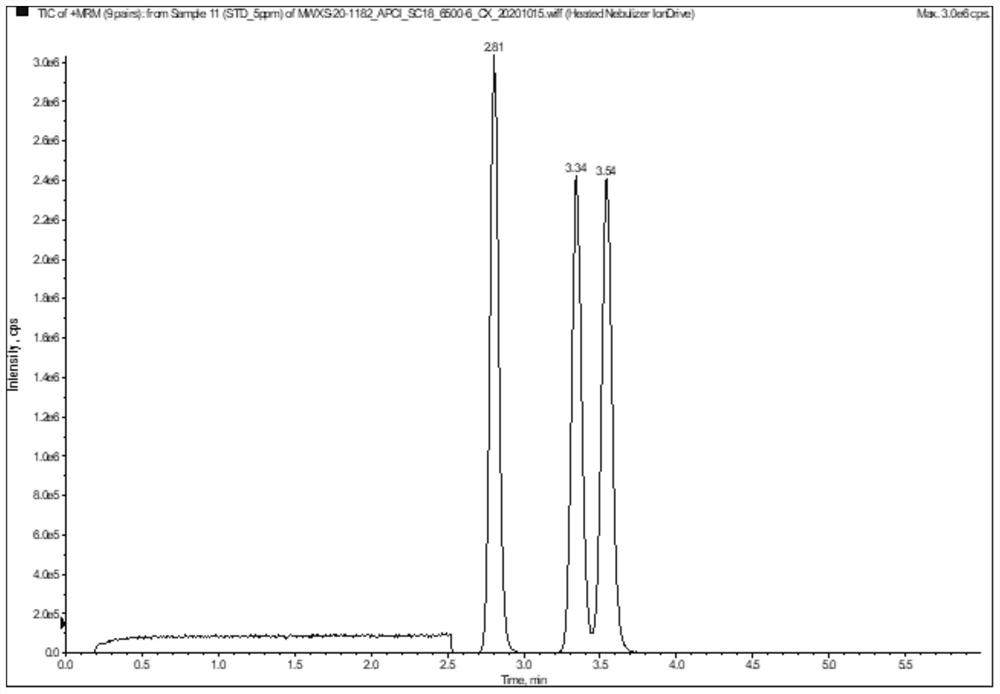
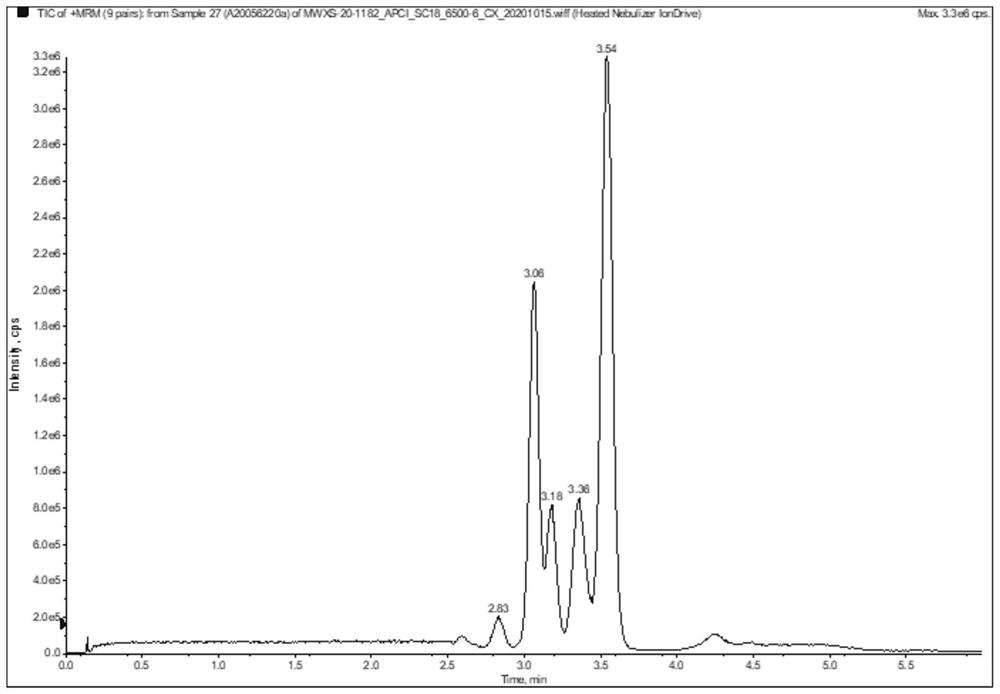
[0043] 3. Optimize the chromatographic method and determine the retention time
[0044] By changing the gradient of the mobile phase and the temperature of the colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com