Azole derivative or pharmaceutically acceptable salt thereof as well as preparation method and application of azole derivative or pharmaceutically acceptable salt thereof
A technology of azole derivatives and derivatives is applied in the field of nuclear transport regulator derivatives and their preparation, which can solve the problems of difficult gastrointestinal toxicity and achieve remarkable effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
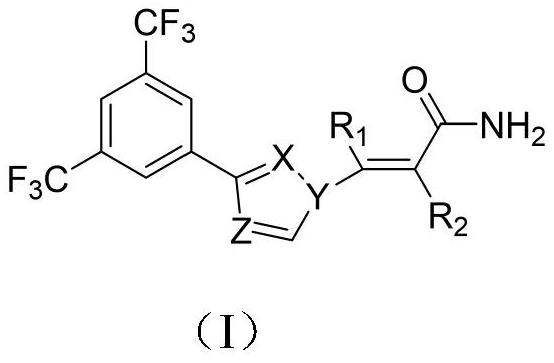
[0039] Embodiment 1 of the present invention is the compound of the present invention or a pharmaceutically acceptable salt thereof, specifically including the preparation process and administration route of the compound.
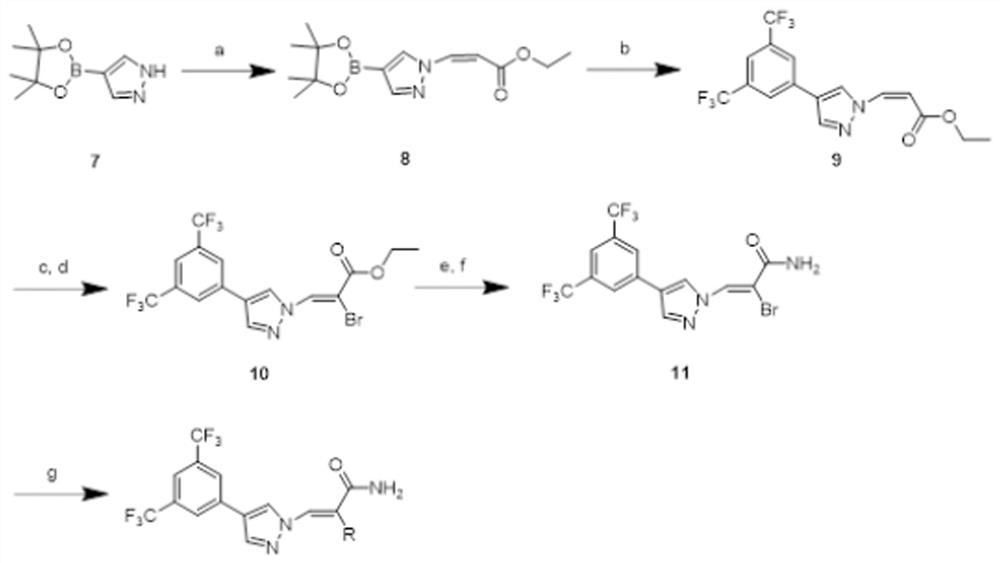
[0040] The following is the synthesis method of the series 1 drug compound and the series 2 drug compound.
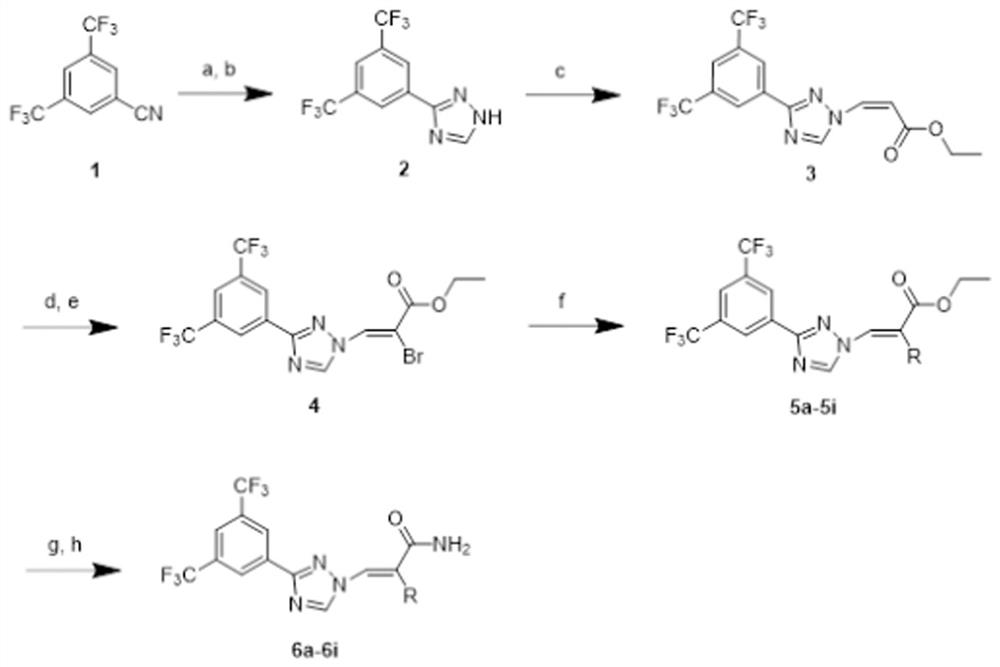
[0041] Such as figure 1 As shown, the route design and synthesis of the series 1 compound of this embodiment. The synthetic method of series 1 compound is: the cyano group of compound 1 generates thioformamide under the effect of sodium hydrosulfide and magnesium chloride, then reacts under the condition of hydrazine hydrate and formic acid to generate triazole 2, and triazole 2 is then reacted in Under the catalysis of triethylenediamine, it is coupled with (Z)-3-iodoethyl acrylate to generate compound 3, and then, the double bond part of compound 3 is added to liquid bromine, and then removed under the action of triethylamine Remove a molecule of...
Embodiment 2
[0087] Embodiment 2 of the present invention is a method for treating a variety of diseases, disorders or conditions related to CRM1 activity in a subject in need thereof, the method comprising administering to the subject in need A therapeutically effective amount of a compound of the invention, or a pharmaceutically acceptable salt or composition thereof.
[0088] The cell line inhibitory activity test is as follows:
[0089] 1. Cell cryopreservation
[0090] (1) After the cells were harvested, they were centrifuged at 1000 rpm for 5 minutes at room temperature and rinsed with PBS.
[0091] (2) Resuspend in 1640 medium containing 7% DMSO and 10% fetal bovine serum.
[0092] (3) Divide into cryopreservation tubes, put them into cell cryopreservation boxes at -80°C overnight, and store them in liquid nitrogen.
[0093] 2. Cell recovery and culture:
[0094] (1) Take the cryopreservation tube out of the liquid nitrogen tank, put it into warm water at 37°C and shake gently t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com