Novel KRAS G12C protein inhibitor as well as preparation method and application thereof
A solvate and independent technology, applied in the field of medicinal chemistry, can solve the problems of high hepatic first-pass effect, excessive dosage, and rapid metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0387] The present invention provides the preparation method of above-mentioned formula I compound, it comprises the following steps:
[0388] 1) Compound I-1 reacts with Compound I-a to obtain Compound I-2;
[0389]
[0390] 2) Compound I-2 undergoes an oxidation reaction to introduce -L-R 1 The reaction and the deprotection reaction of removing PG' to obtain compound I-3;
[0391]
[0392] 3) react compound I-3 and compound I-b to obtain compound I-4;
[0393]
[0394] 4) compound I-4 undergoes a deprotection reaction to remove PG to obtain compound I-5;
[0395]
[0396] 5) Compound I-5 is reacted with compound I-c to obtain a compound of formula I;
[0397]
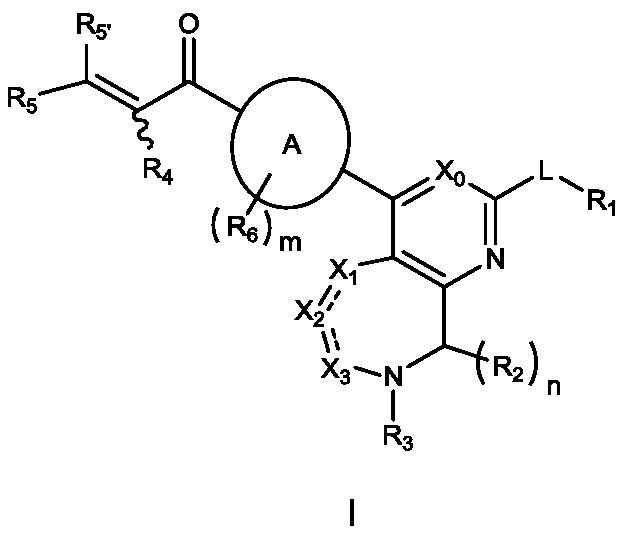
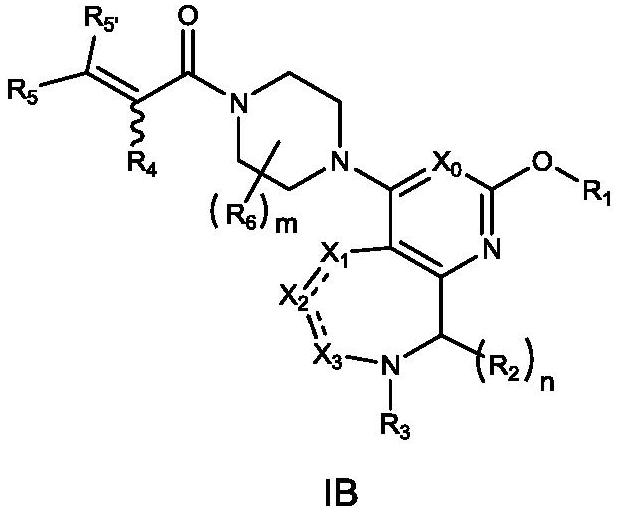
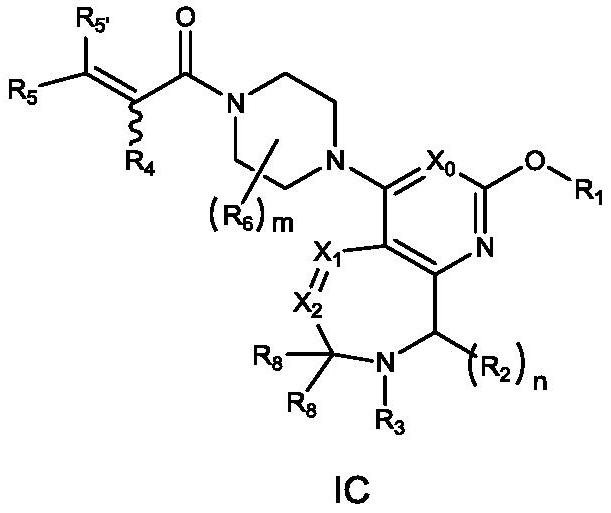
[0398] Wherein: Y is chlorine, bromine, iodine, methanesulfonyloxy, trifluoromethanesulfonyloxy or p-toluenesulfonyloxy; Z is hydroxyl, bromine or chlorine; PG and PG' represent protecting groups; A, L.X 0 、X 1 、X 2 、X 3 , R 1 , R 2 , R 3 , R 4 , R 5 , R 5' , R 6 , m and n are as defined ...
Embodiment 1
[0448] Example 1: 8-Benzyl-2-(methylthio)-6,7,8,9-tetrahydro-5H-pyrimido[4,5-c]azepan-4-yltrifluoro Synthesis of mesylate (Intermediate A).
[0449]
[0450] Step 1: At room temperature, mix benzylamine (Intermediate 1) (50g, 0.47mol) and triethylamine (70.7g, 0.70mol) in acetonitrile (500mL), and add 2-bromoacetic acid dropwise to it under stirring Ethyl ester (78.5g, 0.47mol) in acetonitrile solution (100mL), dripped over about 4h. Filter and wash the filter cake with acetonitrile. The filtrate and acetonitrile washings were combined and concentrated under reduced pressure to obtain the crude N-benzylglycine ethyl ester (Intermediate 2) (76 g, 84% yield), which was directly used in subsequent reactions without purification.
[0451] LC-MS: 194[M+1] + .
[0452]
[0453] Step 2: Mix N-benzylglycine ethyl ester (intermediate 2) (23g, 0.12mol) and triethylamine (14.4g, 0.14mol) in acetonitrile (250mL), and add 5-bromo Ethyl n-valerate (25 g, 0.12 mol). The reaction ...
Embodiment 2
[0465] Example 2: 8-Benzyl-2-(methylthio)-6,7,8,9-tetrahydropyrimidine[4,5-f][1,4]oxazepane-4- Synthesis of triflate (Intermediate B).
[0466]
[0467] Using a synthetic route similar to that of Example 1, 8-benzyl- 2-(Methylthio)-6,7,8,9-tetrahydropyrimidine[4,5-f][1,4]oxazepan-4-yl trifluoromethanesulfonate (intermediate Body B).
[0468] LC-MS: 436[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com