Method for efficiently treating perfluorinated compound polluted water body
A technology for perfluorinated compounds and water bodies, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
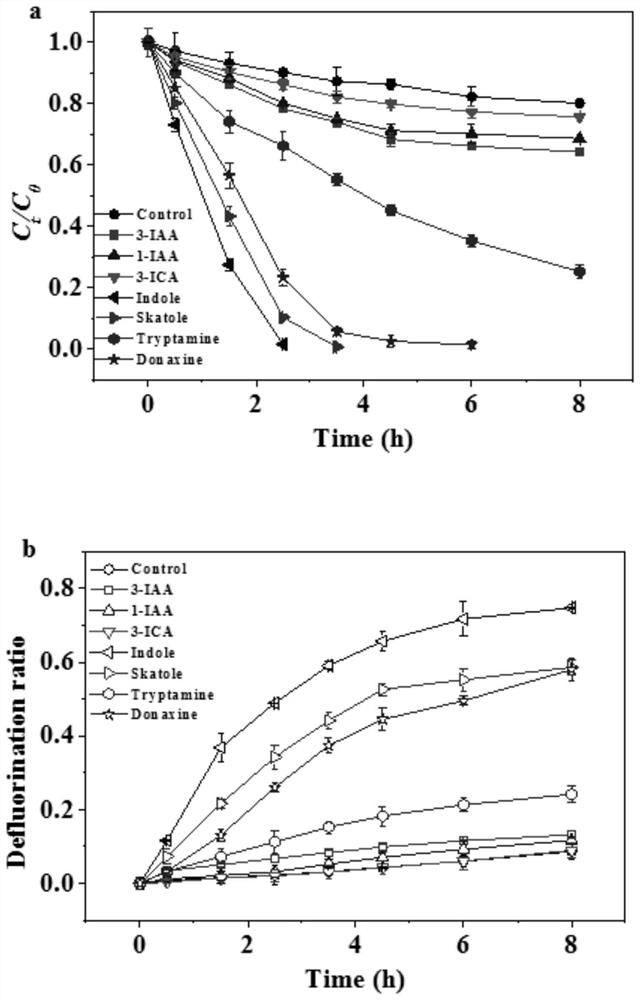
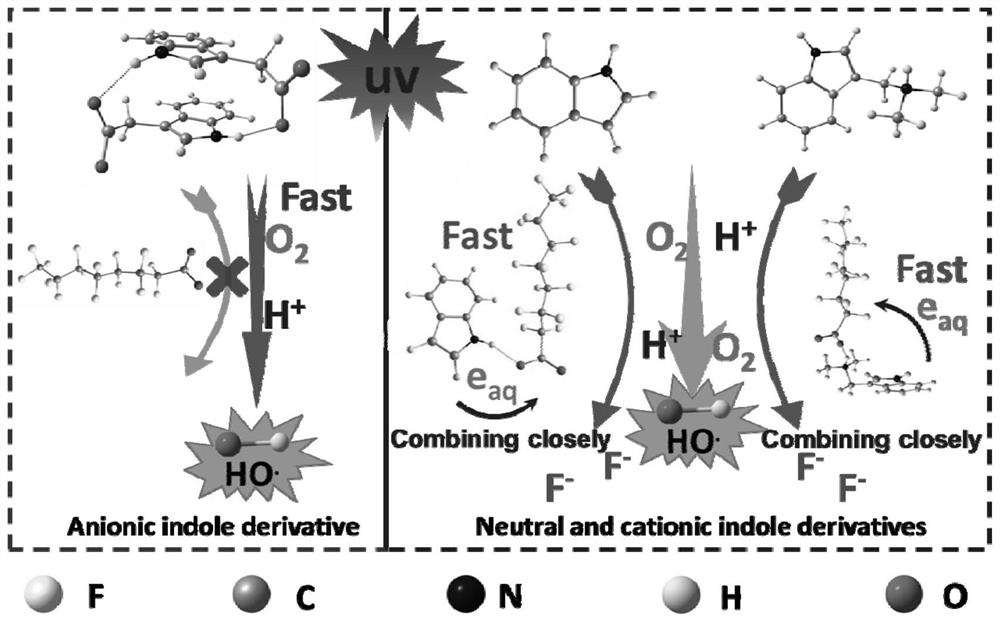
[0040] This example mainly investigates the degradation and defluorination effects of indole derivatives with different electrical properties in water on PFOA, and the specific steps are:
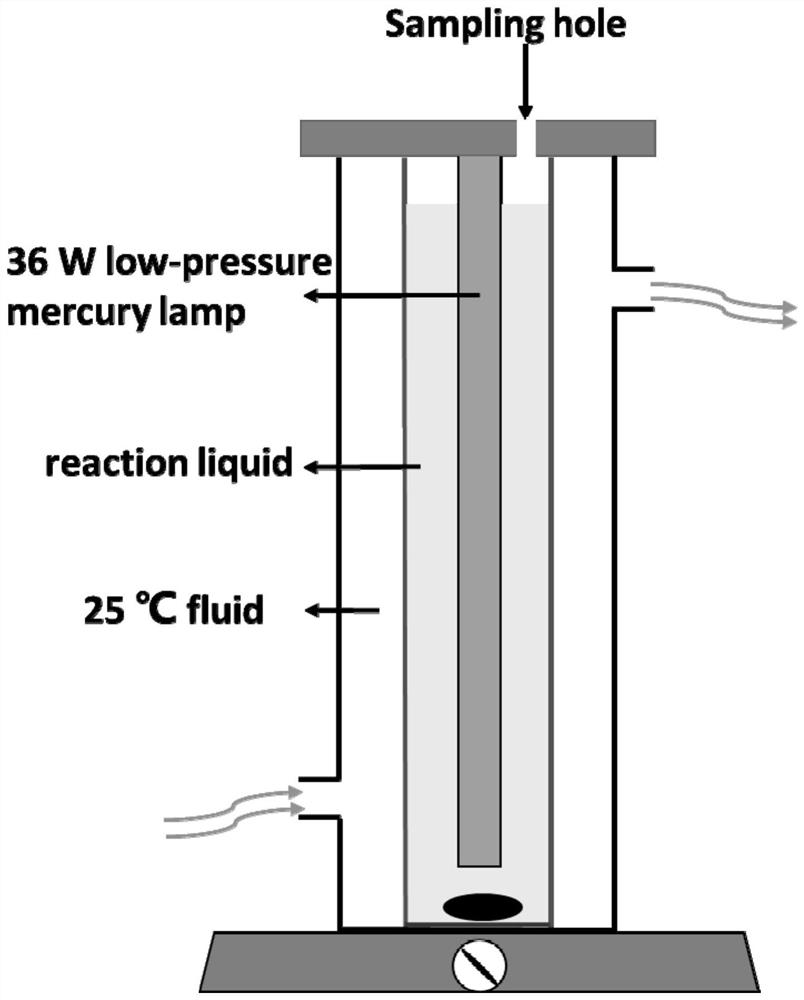
[0041] S10. Prepare 300mL of 1mM 1-indole acetic acid, 3-indole acetic acid, 3-indole propionic acid, 3-indole carboxylic acid, indole, methyl indole, anophylline, tryptamine and The 0.024mM PFOA mixed solution is placed in the quartz light reaction tube (d=41mm; h=400mm), and the 300mL PFOA solution containing only 0.024mM is set as the control group at the same time. The above experiment is carried out in the same reactor, and different mixed solution and control solution to adjust the pH value to 6; wherein, 1-indole acetic acid, 3-indole acetic acid, 3-indole propionic acid, 3-indole carboxylic acid are negatively charged in water, and indole and methyl indole are in The water is electrically neutral, and anophylline and tryptamine are positively charged in water;
[0042] S20, using a...
Embodiment 2
[0045] This example mainly investigates the degradation and defluorination effects of indole derivatives with different electrical properties in water on PFOS, and the specific steps are as follows:
[0046] S10. Put the prepared 300mL mixed solution of 1mM 3-indoleacetic acid, indole, anophylline and 0.024mM PFOS into a quartz photoreaction tube (d=41mm; h=400mm), and set only containing The 300mL PFOS solution of 0.024mM was used as the control group to carry out the above experiment in the same reactor, and adjust the pH value of the different mixed solutions and the control solution to 6; among them, 3-indoleacetic acid is negatively charged in water, and indole in water It is electrically neutral, and agrophylline is positively charged in water;
[0047] S20, using a 36W low-pressure mercury lamp as a light source to perform light reaction on the different mixed solutions obtained in step S10 and the control solution, the reaction temperature is controlled at 25±2° C., an...
Embodiment 3
[0050] This embodiment mainly investigates the influence of pH on the indole system, and its specific steps are;
[0051] S10. Place the mixed solution of 300mL of 1mM indole and 0.024mM PFOA in the quartz photoreaction tube (d=41mm; h=400mm), and mix the mixed solution with different concentrations of NaOH (5mM~1M). The pH value was adjusted to 4, 6, 8, 10 respectively;
[0052] S20. Use a 36W low-pressure mercury lamp as a light source to perform light reaction, the reaction temperature is controlled at 25±2° C., and the reaction time is 8 hours. The sampling time is set to 0h, 0.5h, 1.5h, 2.5h, 3.5h, 4.5h, 6h, 8h respectively. The sample is divided into two parts, the remaining PFOA content is measured by LC-MS / MS, and the generated F ion content is measured by ion chromatography (IC), so as to calculate the degradation rate and defluorination rate of PFOA. The specific degradation curve and defluorination curve are respectively Such as Figure 5 (a) and Figure 5 (b) s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com