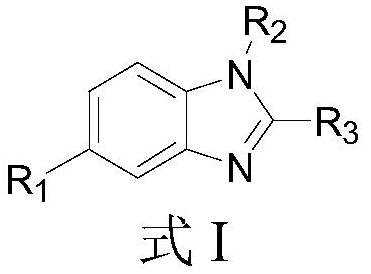
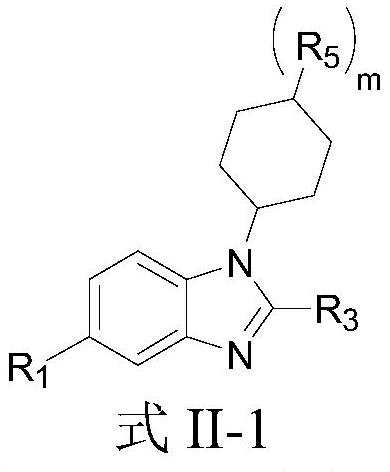
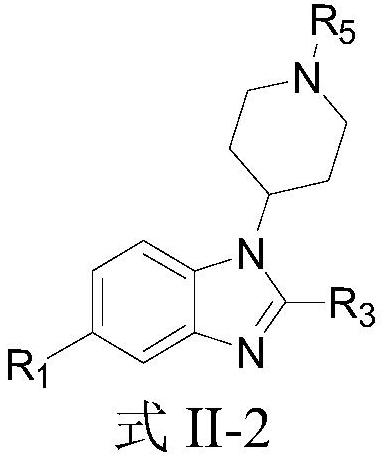
EP300/CBP inhibitor
A deuterated, selected technology, applied in the field of pharmaceutical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 100
[0142] Example 100 compound (1S, 4S, 5R)-3-(3,4-difluorophenyl)-4-(5-(3,5-dimethylisoxazol-4-yl)-1-( (1r,4S)-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexa-2 -Synthesis of ketone (compound 100):
[0143] (1R,2S,5S)-3-tert-butyl-2-methyl-6,6-dimethyl-4-oxo-3-azabicyclo[3.1.0]hexane-2,3-di Synthesis of Carboxylate (Compound 100-2):
[0144]
[0145] Starting material 100-1 (269 mg, 1 mmol), sodium periodate (850 mg, 4 mmol) was dissolved in 8 mL of acetonitrile and 8 mL of water. Under cooling in an ice-water bath, 5 mg of ruthenium trichloride was added and reacted for 5 hours. It was poured into water, extracted with EA, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and column chromatographed to obtain 112 mg of the product (intermediate 100-2), with a yield of 40%.
[0146] Ms:284(M+H + )
[0147] Synthesis of (1R,2S,5S)-6,6-dimethyl-4-oxo-3-azabicyclo[3.1.0]hexa-2-carboxylic acid methyl ester (in...
Embodiment 101
[0163] Example 101 Compound 2-(3,4-difluorophenyl)-1-(5-(3,5-dimethylisoxazol-4-yl)-1-((trans)-4-methan Synthesis of oxycyclohexyl)-1H-benzo[d]imidazol-2-yl)-2-azabicyclo[3.1.0]hexan-3-one (101):
[0164] Synthesis of 4-(2-hydroxyethyl)-1,3,2-dioxothioalkane-2,2-dioxide (Intermediate 101-1):
[0165]
[0166] Add 1,2,4-tributanol (10.0g, 94.2mmol) into pyridine (15.2mL) and acetonitrile (100mL), add thionyl chloride (34.4mL) dropwise at 0°C, and the reaction solution is The stirring reaction was continued for 15h. After the reaction was complete, ethyl acetate (20 mL) and 0.1 M hydrochloric acid solution (10 mL) were added, and extracted with ethyl acetate (10 mL×2). The organic phase was washed once with saturated brine (20 mL), dried over anhydrous sodium sulfate, and filtered to obtain a filtrate. The solvent was distilled off from the filtrate under reduced pressure to obtain 14.5 g of an oily substance.
[0167] The above 14.5g oil was dissolved in acetonitrile (20...
Embodiment 102
[0193] Example 102 compound (1S,5S)-2-(3,4-difluorophenyl)-1-(5-(3,5-dimethylisoxazol-4-yl)-1-((trans Formula)-4-(methoxy-d3)cyclohexyl)-1H-benzo[d]imidazol-2-yl)-2-azabicyclo[3.1.0]hexane-3-one (102) synthesis:
[0194] Synthesis of (S)-4-(2-hydroxyethyl)-1,3,2-dioxothioalkane-2,2-dioxide (Intermediate 102-1):
[0195]
[0196] Add (S)-1,2,4-tributanol (10.0g, 94.2mmol) into pyridine (15.2mL) and acetonitrile (100mL), add thionyl chloride (34.4mL) dropwise at 0°C, and react The solution was stirred and reacted for 15 h at room temperature. After the reaction was complete, ethyl acetate (20 mL) and 0.1 M hydrochloric acid solution (10 mL) were added, and extracted with ethyl acetate (10 mL×2). The organic phase was washed once with saturated brine (20 mL), dried over anhydrous sodium sulfate, and filtered to obtain a filtrate. The solvent was distilled off from the filtrate under reduced pressure to obtain 14.2 g of an oily substance.
[0197] The above 14.2g oil was d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com