Amphiphilic graft copolymer and application thereof
A graft copolymer and hydrophilic polymer technology, applied in the field of pharmaceutical preparations, can solve the problems of lack of reversibility of intelligent responsive micelles, and achieve the effects of improving stability and targeting efficiency and improving safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Synthesis of γ-PGA-NH-PHis
[0050] (1) γ-PGA-NH 2 Synthesis of γ-PGA: Weigh 100 mg of γ-PGA, add 2 mL of ultrapure water, stir to dissolve, add 345.06 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC.HCl) , 207.18mg of N-hydroxysuccinimide (NHS), activated at room temperature for 30min. Weigh 270.45 mg of ethylenediamine (EDA) and add it into the activated γ-PGA solution, and stir at room temperature for 12 hours. After the reaction, the reaction solution was dialyzed with a dialysis bag with a molecular weight cut-off of 3.5 KDa, and freeze-dried for 48 hours to obtain a white loose solid, namely γ-PGA-EDA.
[0051] (2) Synthesis of γ-PGA-NH-PHis: Weigh 67.62 mg of PHis (16), 11.50 mg of EDC.HCl, and 6.9 mg of NHS, dissolve in 2 mL of dimethyl sulfoxide (DMSO), and activate at room temperature for 30 min. Weigh 41.43 mg of γ-PGA-EDA, add 300 μL of ultrapure water to dissolve, dilute to 2 mL with DMSO, add to the activated PHis solution, and ...
Embodiment 2
[0088] Synthesis of PVA-PHis: 20 mg of PHis (16), 6.82 mg of EDC.HCl, and 4.34 mg of DMAP were dissolved in 5 mL of 0.1 M MES buffer (pH=5.8), and activated at room temperature for 30 min. Weigh 10 mg of PVA and dissolve it in 2 mL of MES buffer, add it into the activated PHis solution, and stir at room temperature for 24 hours. The reaction solution was dialyzed and freeze-dried to obtain a white solid, namely PVA-PHis.
[0089] Preparation of micelles: Dissolve an appropriate amount of PVA-PHis in PBS solution with pH 7.4 to form micelles by self-assembly.
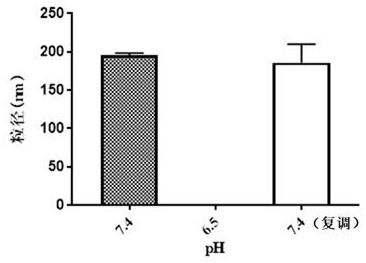
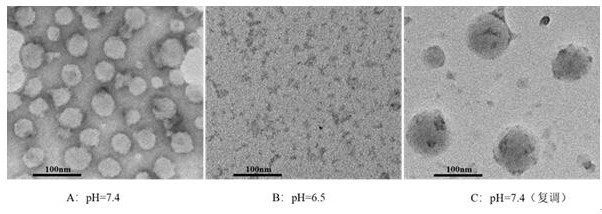
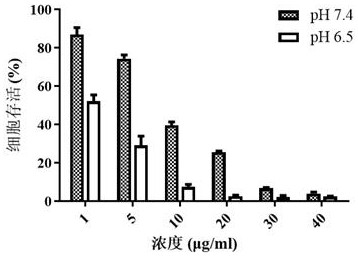
[0090] Measure the hydrated particle size of the micellar solution obtained in Example 5 with the technique of dynamic light scattering, adjust the pH of the solution to 6.5, and after standing for half an hour, measure its hydrated particle size again, then adjust the pH of the solution to 7.4, and let it stand After half an hour, measure its hydrated particle size again. The results of hydrated particle size under di...
Embodiment 3
[0095] Synthesis of PEI-PHis: 20 mg of PHis (16), 6.82 mg of EDC.HCl, and 4.08 mg of NHS were dissolved in 5 mL of 0.1 M MES buffer (pH=5.8), and activated at room temperature for 30 min. Weigh 15 mg of PEI and dissolve it in 2 mL of MES buffer, add it into the activated PHis solution, and stir at room temperature for 24 h. The reaction solution was dialyzed and freeze-dried to obtain a white solid, namely PEI-PHis.
[0096] Preparation of micelles: Dissolve an appropriate amount of PEI-PHis in PBS solution with pH 7.4 to form micelles by self-assembly.
[0097] The hydrated particle size of the obtained micellar solution was measured by dynamic light scattering method, the pH of the solution was adjusted to 6.5, and after standing for half an hour, the hydrated particle size was measured again, and the pH of the solution was readjusted to 7.4, and after standing for half an hour , and measure its hydrated particle size again. The results of hydrated particle size under diff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com