A kind of drug-loaded nanoparticle with targeted core-shell structure and preparation method thereof
A drug-loaded nanometer, core-shell structure technology, used in pharmaceutical formulations, drug combinations, antitumor drugs, etc., can solve the problems of cytotoxicity, cell membrane damage, insufficient metabolic properties of inorganic nanocarriers, etc., to improve the enrichment and retention. , improve stability, avoid degradation and the effect of chemotherapeutic drug leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
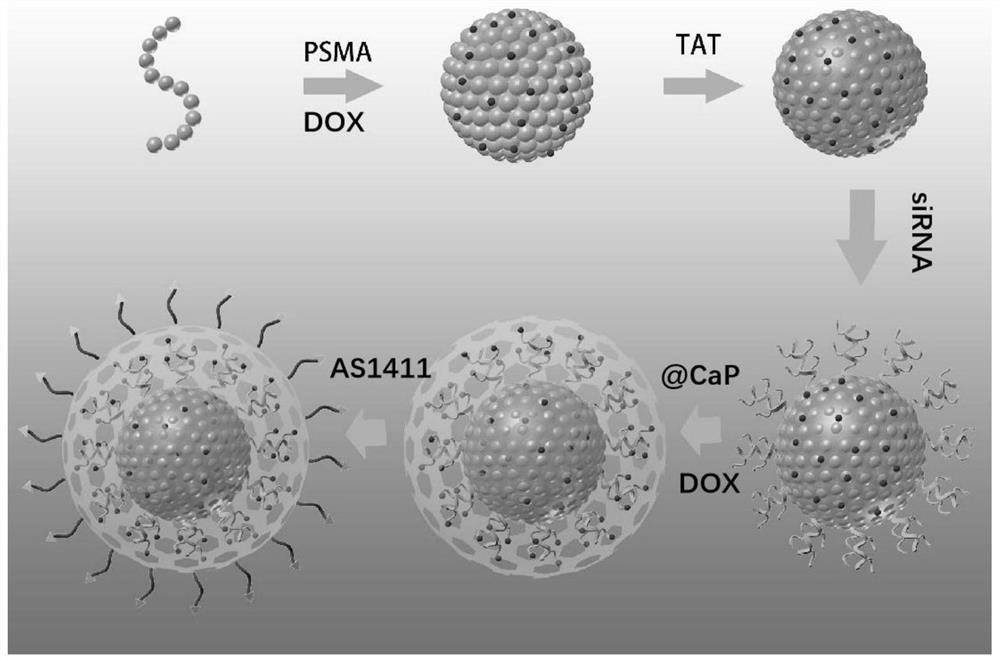
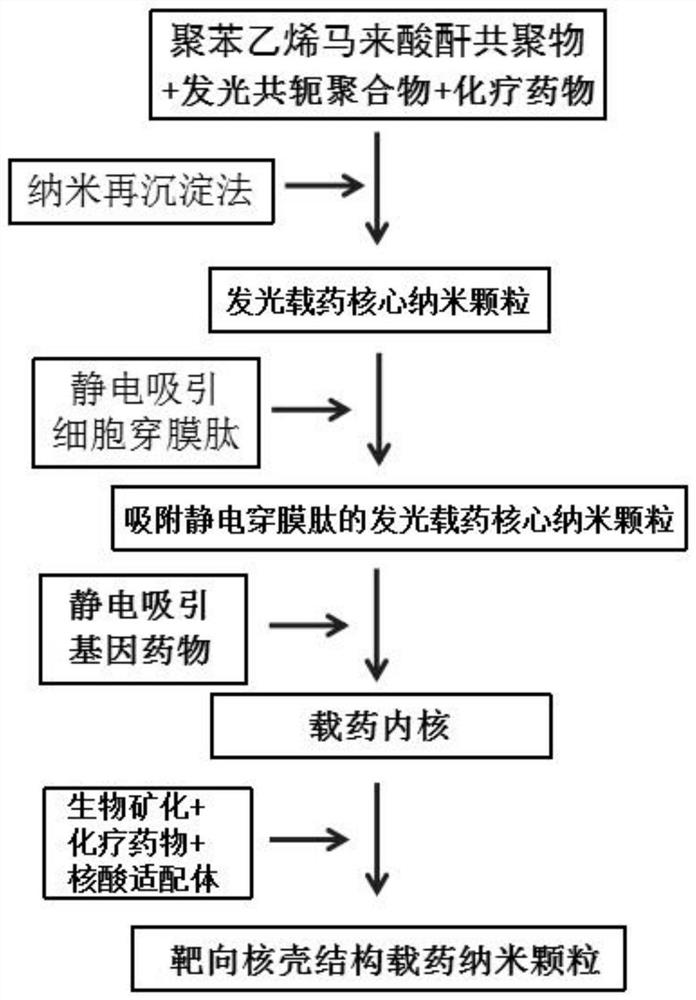
[0059] The preparation method provided by the invention comprises the following steps:
[0060] (1) Dissolving chemotherapeutic drugs, light-emitting conjugated polymers and polystyrene-maleic anhydride copolymers in an organic solvent, under the action of ultrasound, mixing the resulting mixed solution with water, then distilling off the organic solvent and removing the remaining Filtrating the solution to obtain a luminescent drug-loaded core nanoparticle solution;
[0061] (2) mixing the cell-penetrating peptide, the luminescent drug-loaded core nanoparticle solution and the buffer solution to obtain a cell-penetrating peptide-modified luminescent drug-loaded core nanoparticle solution;
[0062] (3) mixing the gene drug and the luminescent drug-loaded core nanoparticle solution modified by the cell-penetrating peptide to obtain a drug-loaded core nanoparticle solution;
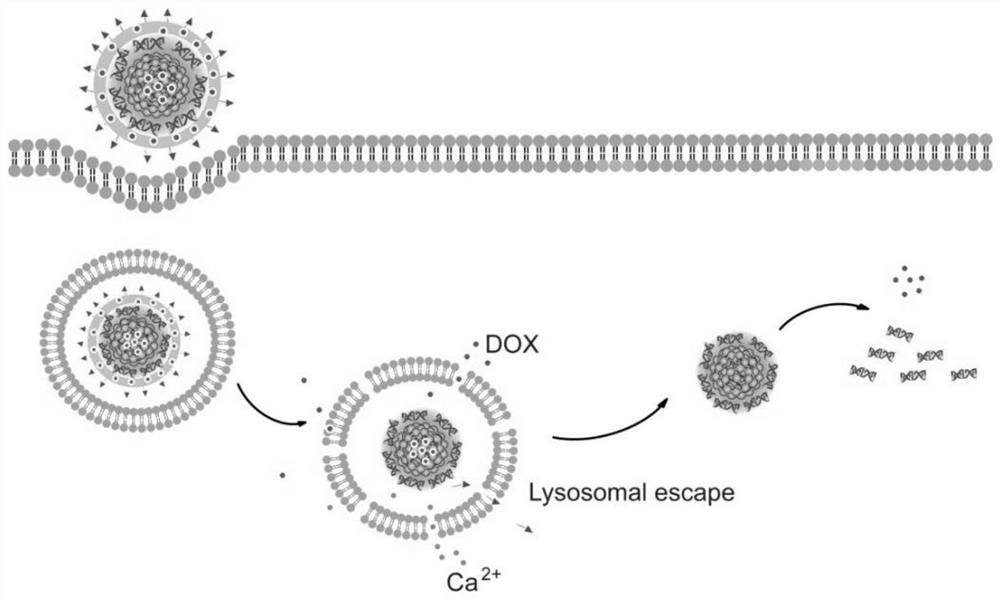
[0063] (4) mixing calcium chloride, nucleic acid aptamer, chemotherapeutic drugs and drug-loaded core n...
Embodiment 1
[0082] Preparation of luminescent drug-loaded core nanoparticles (D / CPNPs) solution:
[0083] Preparation of core luminescent drug-loaded nanoparticles by nano-reprecipitation method, the steps are as follows:
[0084] (1) The chemotherapeutic drug doxorubicin (DOX), luminescent conjugated polymer (CN-PPV) and polystyrene maleic anhydride copolymer (PSMA) were dissolved in tetrahydrofuran to obtain DOX solution, CN-PPV solution and PSMA solution, the final concentration is 1mg / mL, as the original solution;
[0085] (2) Mix the DOX solution, CN-PPV solution and PSMA solution, and dilute with tetrahydrofuran to obtain a mixed solution, the mass concentrations of DOX, CN-PPV and PSMA in the mixed solution are successively 10 μg / mL and 25 μg / mL and 5 μg / mL.
[0086] (3) Under the action of ultrasound, quickly inject the mixed solution into ultrapure water to obtain a nanoparticle mixed system, and continue to sonicate the nanoparticle mixed system for 1 min.
[0087] (4) Heatin...
Embodiment 2
[0090] Preparation of Luminescent Drug-loaded Core Nanoparticles (D / CPNPs) Solution
[0091] The nano-reprecipitation method is used to prepare luminescent drug-loaded core nanoparticles, and the steps are as follows:
[0092] (1) Dissolve the chemotherapeutic drug doxorubicin (DOX), luminescent conjugated polymer (CN-PPV) and polystyrene maleic anhydride copolymer (PSMA) in dimethyl sulfoxide to obtain DOX solution, CN-PPV -PPV solution and PSMA solution, the final concentration is 1mg / mL, as the original solution;
[0093] (2) Dilute with dimethyl sulfoxide after mixing the DOX solution, CN-PPV solution and PSMA solution to obtain a mixed solution, the mass concentration of DOX, CN-PPV and PSMA in the mixed solution is 20 μg / mL successively , 50 μg / mL and 10 μg / mL.
[0094] (3) Under the action of ultrasound, quickly inject the mixed solution into ultrapure water to obtain a nanoparticle mixed system, and continue to sonicate the nanoparticle mixed system for 2 minutes.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com