Method for producing intermediate useful for synethesis of SGLT inhibitor
A technology of compounds and catalysts, applied in the field of preparation of intermediates, can solve problems such as safety management burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0315] Now, the present invention will be described in more detail with reference to the following examples. These examples are provided for illustrative purposes only and should not be construed as limiting the scope and spirit of the invention.
[0316] The abbreviations described in the examples below have the following meanings.
[0317] -DMF:N,N-Dimethylformamide
[0318] -EtOH: ethanol
[0319] -Et 3 SiH: Triethylsilane
[0320] -MC: dichloromethane
[0321] -MC / AN: dichloromethane and acetonitrile
[0322] -NaOH: sodium hydroxide
[0323] -RT or rt: room temperature
[0324] Reaction Pattern Diagram
[0325]
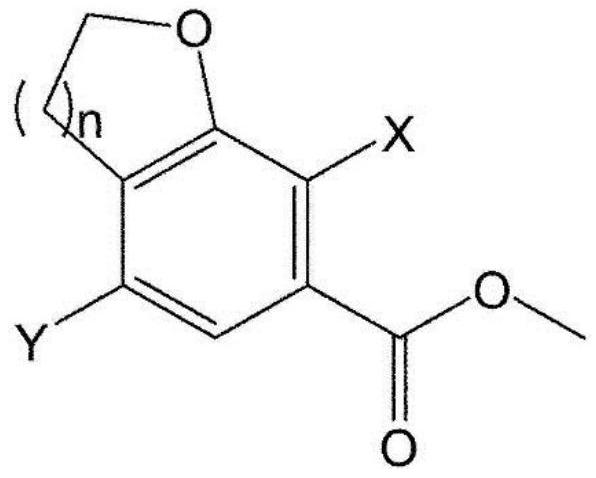
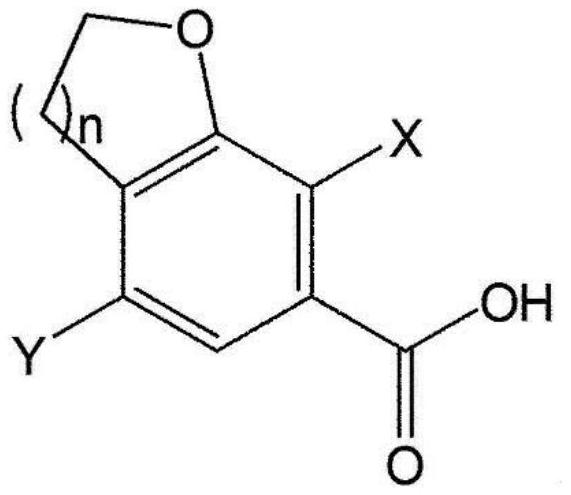
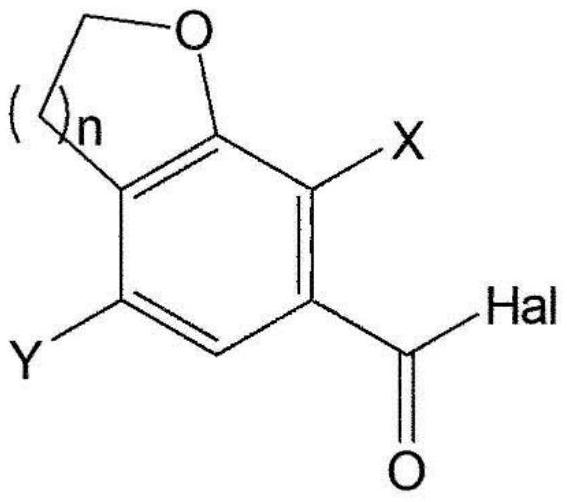
[0326] The first step: 4-bromo-7-chloro-2,3-dihydrobenzofuran-6-carboxylic acid (compound 2)
[0327]
[0328]Add 4N sodium hydroxide (51.4 mL, 205.8 mmol) to 4-bromo-7-chloro-2,3-dihydrobenzofuran-6-carboxylic acid methyl ester (Compound 1) (20.0 g, 68.6 mmol) in ethanol (200 mL). The mixture was stirred at room temperature for 2 hours, and after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com