Purpose of DNA tetrahedron in preparation of medicine capable of treating renal injury
A technology of tetrahedron and kidney damage, which can be applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., and can solve problems such as obvious side effects and application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis and identification of embodiment 1 tFNAs
[0027] 1. Synthesis method
[0028] Dissolve the four DNA single strands (S1, S2, S3, S4) in TM Buffer (10mM Tris-HCl, 50mM MgCl 2 , pH=8.0), the final concentration of the four DNA single strands was 1000nM, mixed thoroughly, rapidly heated to 95°C for 10 minutes, and then rapidly cooled to 4°C and maintained for more than 20 minutes to obtain tFNAs.
[0029] The sequences of the four single strands (5'→3') are as follows:
[0030]
[0031] 2. Identification
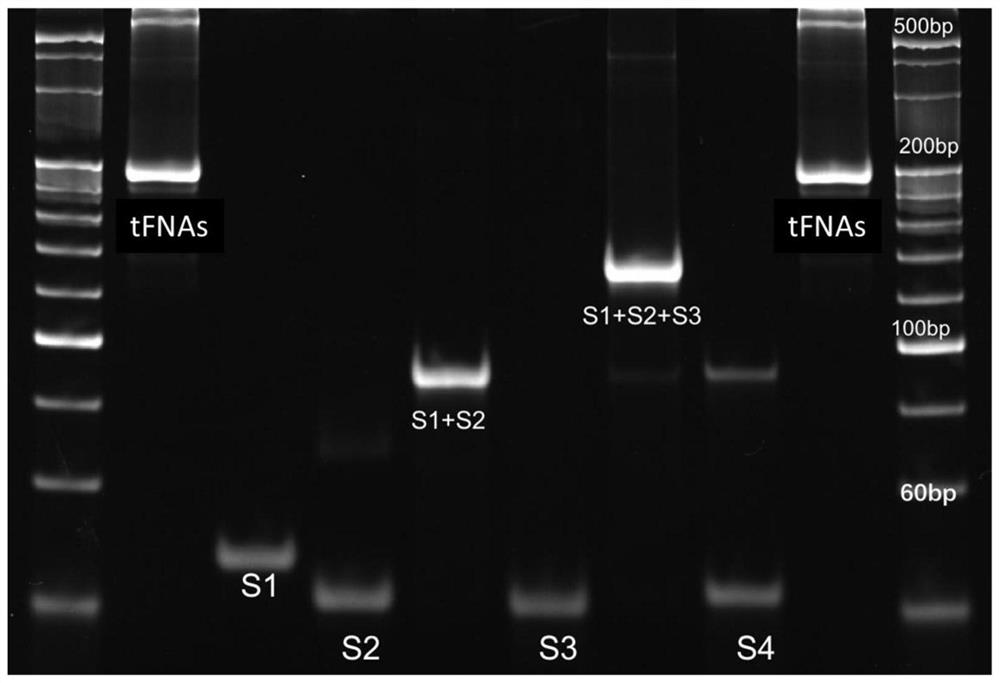
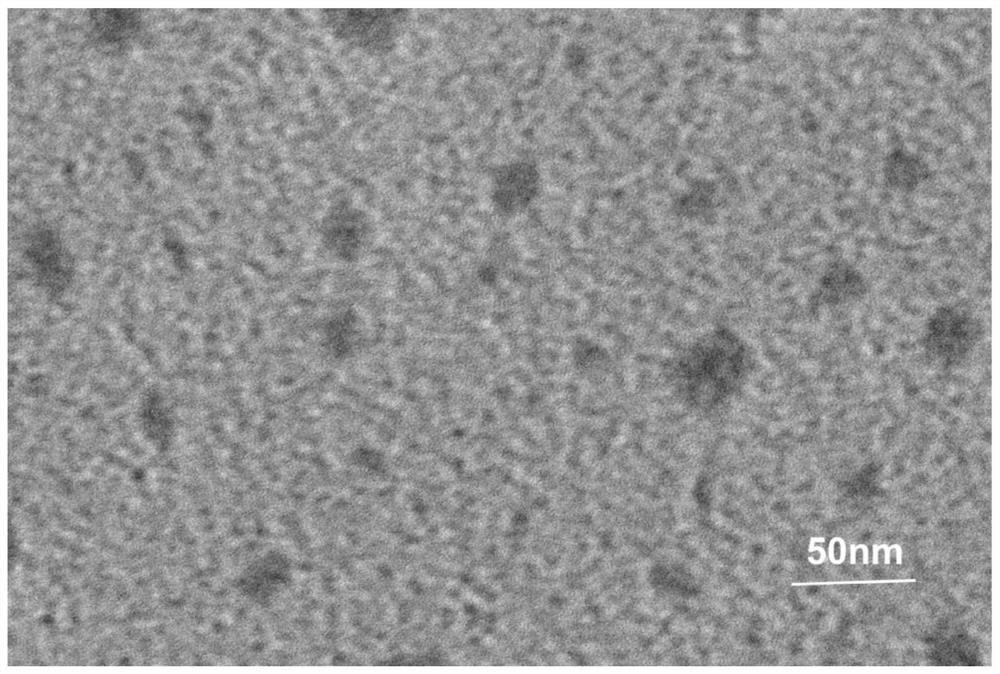
[0032] After synthesis of tFNAs, SDS-PAGE electrophoresis shows that tFNAs is about 200bp, it can be considered that tFNAs have been successfully synthesized ( figure 1 ). Using transmission electron microscopy, the tetrahedral structure of tFNAs characteristics can be seen under the microscope ( figure 2 ).
[0033] The present invention will be further described in the form of experimental examples below.
experiment example 1
[0034] Experimental example 1 Distribution of tFNAs in mice
[0035] 1. Method
[0036] The fluorescent molecule Cy5 was used to label tFNAs, half an hour after intravenous injection (injection volume: 40 microliters) of Balb / c mice, each tissue and organ was collected, and the fluorescence of each tissue and organ was detected by a small animal imaging system.
[0037] 2. Results
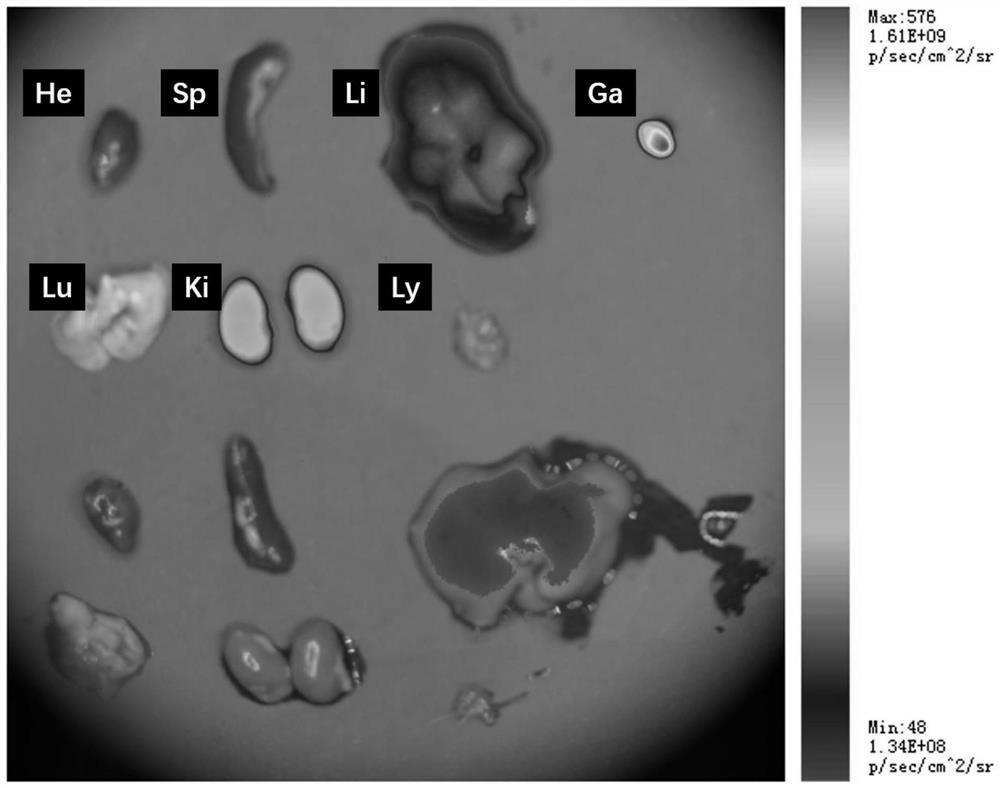
[0038] The fluorescence intensity of cy5-tFNAs in the mouse liver, kidney and gallbladder was stronger, among which the fluorescence intensity of tFNAs in the gallbladder was the highest, followed by the kidney ( image 3 ).
[0039] The results of this experimental example show that tFNAs can be enriched in the kidney, which is conducive to its therapeutic effect.
experiment example 2
[0040] Experimental example 2 Animal experiments on the curative effect of tFNAs on kidney injury
[0041] 1. Method
[0042] Glycerol (8 mg / kg) was intramuscularly injected into the hind limbs of balb / c mice to establish a rhabdomyolysis-induced acute kidney injury mouse (AKI mouse) model. At the same time, the healthy mice injected with saline into their hind limbs were regarded as the sham operation group; the mice without any treatment were regarded as the blank group. 2 hours after modeling, inject tFNAs into AKI mice (40 μl, concentration: 1 μmol / L) as the tFNAs group, inject normal saline (40ul) into the tail vein of AKI mice (for the AKI group) and the mice of the sham operation group . After 24 hours, the kidney tissues of the mice were collected, fixed and made into paraffin sections for HE staining.
[0043] 2. Results
[0044] The result is as Figure 4 As shown, compared with normal renal histopathology, renal tubular epithelial cells in the AKI group were sw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com