Monosulfuron-methyl derivative and preparation method thereof, herbicide and weeding method
A technology of monosulfuron-methyl and its derivatives, which is applied in the field of monosulfuron-methyl derivatives and their preparation. It can solve the problems of not being able to use antifly, easy to block spray pipes, and poor water solubility of monosulfuron-methyl, and achieve excellent removal effect , Promote soil remediation, excellent herbicidal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The embodiment of the present invention also provides a preparation method of monosulfuron-methyl derivatives, comprising: reacting monosulfuron-methyl with a reaction material capable of forming a quaternary ammonium cation to form the monosulfuron-methyl derivatives.
[0046] Specifically, the reaction is carried out by mixing monosulfuron-methyl, an organic solvent, ammonia or an organic amine; wherein, the organic solvent is an aprotic solvent; for example, the organic solvent includes but is not limited to acetonitrile, acetone, 1,4-dioxane At least one of ring, dichloromethane, chloroform, 1,2-dichloroethane and tetrahydrofuran; the reaction temperature is 0-50°C, preferably 15-35°C, and the reaction time is 2-24 hours. Adoption of the above reaction conditions is beneficial to the progress of the reaction and to the improvement of the yield and purity of the monosulfuron-methyl derivatives.
[0047] Further, after the reaction is finished, the reaction mixture so...
Embodiment 1
[0057] The embodiment of the present invention provides a synthesis method of monosulfuron-methyl (N-[2'-(4-methyl)-pyrimidinyl]-2-nitrobenzenesulfonylurea) ammonium salt, comprising:
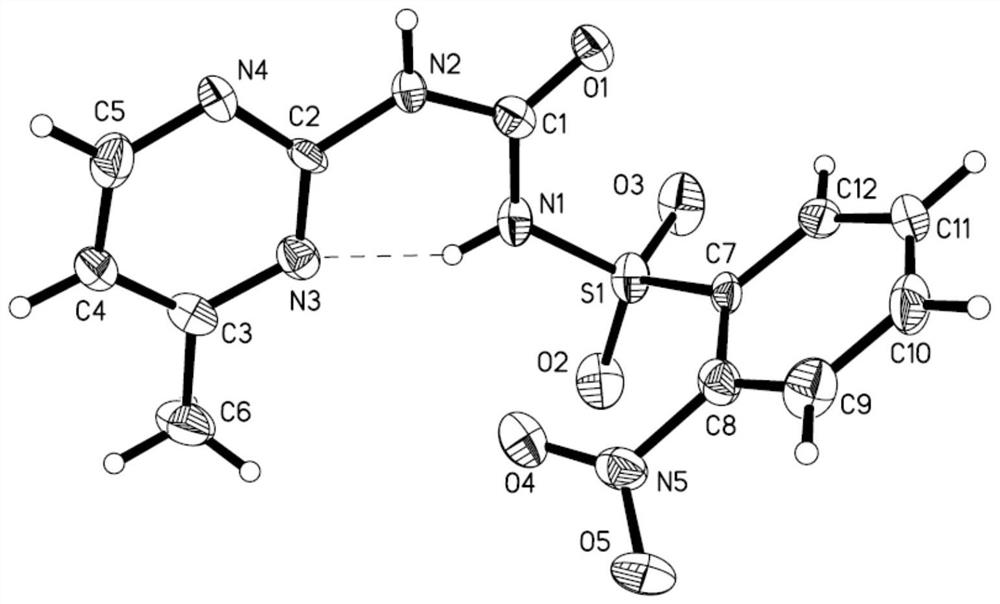
[0058] Weigh 0.05mol monosulfuron-methyl (16.85g) and 0.055mol (7.7g) of 25% ammonia water into 200mL of dichloromethane, stir at 25°C for 2 hours, after the reaction, add 200mL of water; The layer was precipitated to obtain a light yellow powder, and recrystallized with methanol to obtain a white needle-like crystal, which was the monosulfuron-methyl ammonium salt obtained in the embodiment of the present invention. The melting point is 175-176°C, and the yield: 96%.
[0059] The monosulfuron-methyl ammonium salt obtained by this embodiment is characterized (see Figure 2-Figure 4 ): Elemental analysis measured value is C:40.43%, H:4.69%, N:21.78%; Theoretical calculation is C:40.41%, H:4.70%, N:21.75%, all within the experimental error range; 'HNMR( DMSO-d6)δ values were 2.29(s,3H, Pyrimi...
Embodiment 2
[0061] The embodiment of the present invention provides a synthesis method of monosulfuron-methyl (N-[2'-(4-methyl)-pyrimidinyl]-2-nitrobenzenesulfonylurea) methylamine salt, comprising:
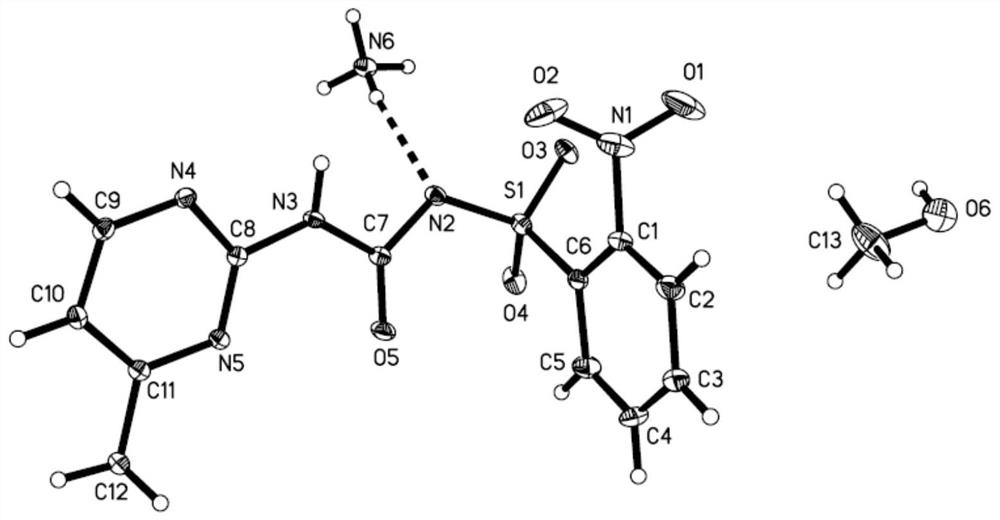
[0062] Weigh 0.05mol monosulfuron-methyl (16.85g) and 8 milliliters of 40% methylammonia aqueous solution into 200 mL of dichloromethane, stir at room temperature for 2 hours, add 200 mL of water to dissolve; separate liquid, and precipitate the water layer to obtain a white solid , recrystallized with methanol to obtain white crystals, which is the monosulfuron-methyl salt of the present invention. The melting point is 173-175°C, and the yield: 95%.
[0063] To characterize it (see Figure 5 ), the characterization data are as follows: the elemental analysis measured values are C: 42.39%, H: 4.28%, N: 22.92%; theoretical calculations are C: 42.39%, H: 4.38%, N: 22.81%, all within the experimental error range ; 1 HNMR(DMSO-d6)δ values are 2.29(s,3H,pyrim-CH 3 ),2.33(s,3H,CH 3 NH 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com