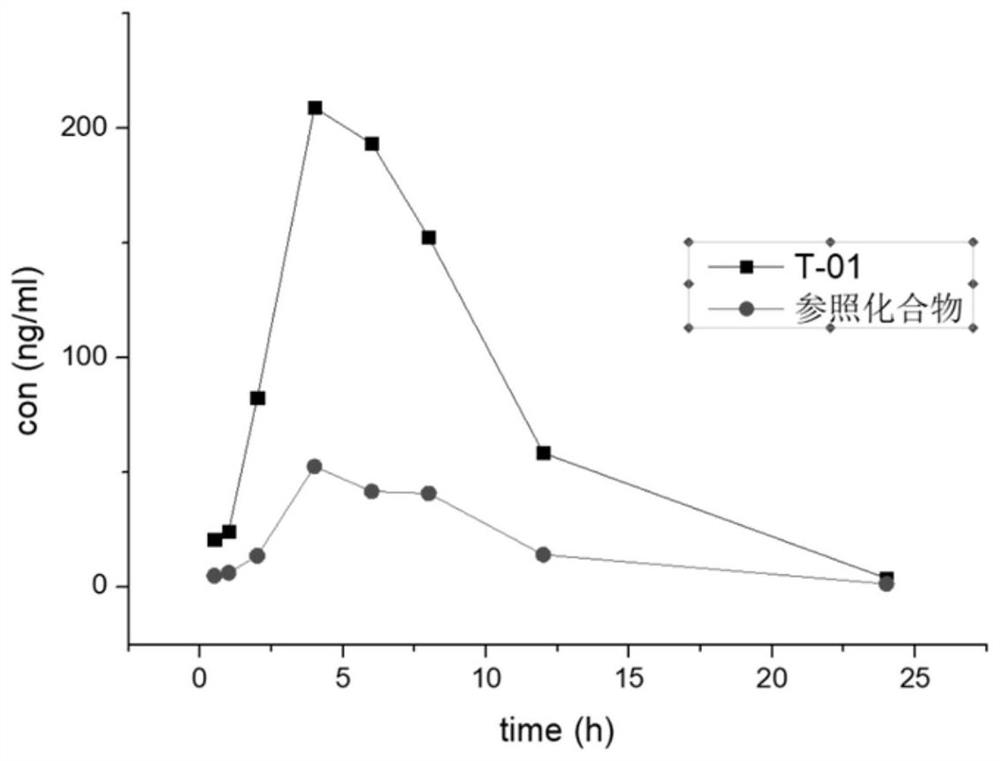
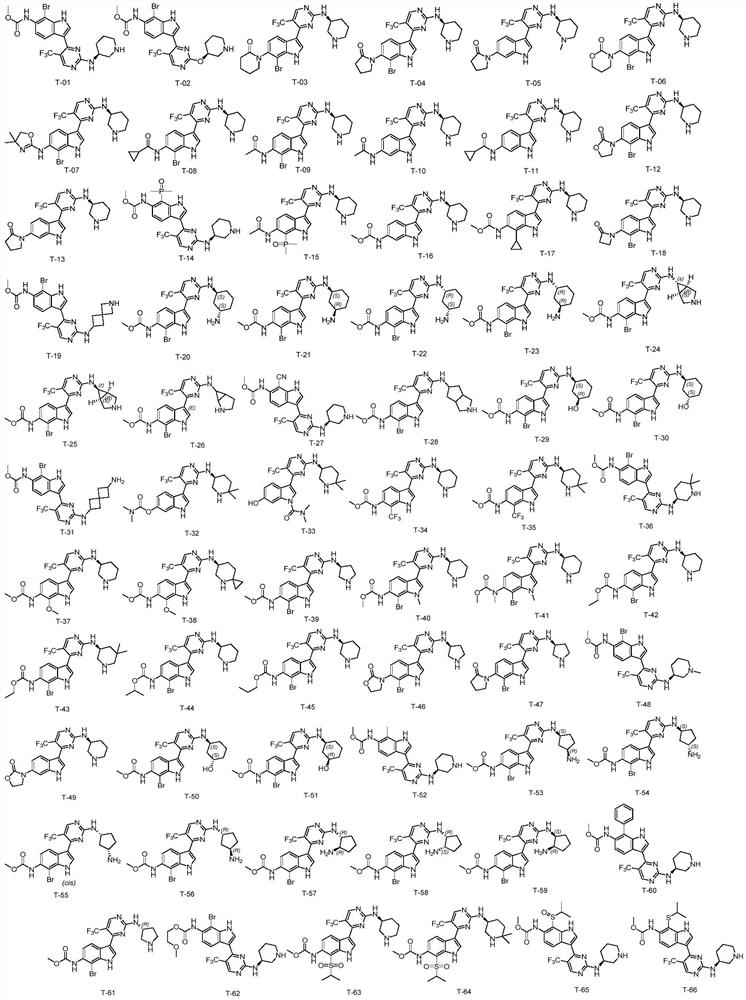
Compound used as CDK7 kinase inhibitor and application thereof
A kind of kinase inhibitor and compound technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0188] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula I of the present invention or its crystal form, pharmaceutically acceptable salt, hydrate or solvate substances are mixed to form a pharmaceutical composition.
[0189] The present invention also provides a treatment method, which comprises the steps of: administering the compound of formula I described in the present invention, or its crystal form, pharmaceutically acceptable salt, hydrate or solvate, or administering The pharmaceutical composition of the present invention is used for inhibiting CDK7.
[0190] Compared with the prior art, the present invention has the following main advantages:
[0191] (1) The compound of the present invention has excellent inhibitory ability to CDK7 kinase;
[0192](2) The compound of the present invention has lower toxic and side effects...
Embodiment
[0197] The technical solution of the present invention will be further described below, but the protection scope of the present invention is not limited thereto.
[0198] Synthesis of Intermediate 1
[0199] The structural formula of intermediate 1 is as follows:
[0200]
[0201] The experimental process is as follows:
[0202] The synthetic route is as follows:
[0203]
[0204] Synthetic steps:
[0205] (1) Synthesis of compound 2
[0206] Dissolve 175g (1.0eq) of compound 1 in tert-butanol, stir at room temperature, add triethylamine to the reaction solution, the solution changes from clear yellow to clear reddish brown, under nitrogen protection, slowly add DPPA (1.5eq) dropwise, and Slowly raise the temperature of the reaction system to 80°C, and at the same time, a large number of bubbles will be generated. After the bubbles were reduced, the reaction was continued at 80°C for 3 hours, then an aqueous solution of sodium carbonate was added to quench the react...
Embodiment T-01
[0265] The synthetic compound of the present invention:
[0266]
[0267] The experimental process is as follows:
[0268] The synthetic route is as follows:
[0269]
[0270] Synthetic steps:
[0271] (1) Synthesis of Compound 2
[0272] Add 11.5g (1.0eq) compound 1, 18.4g (1.4eq) DPPA, 8.7g (1.8eq) triethylamine, 100ml dioxane in a 250ml three-necked flask, react at 80°C under nitrogen protection for 1h, add 3.5ml ( 2.0eq) methanol, continue to react for 3h. TLC monitored the reaction to complete. Water was added, extracted with EA, and purified by column to obtain 9.2 g of white solid.
[0273] (2) Synthesis of compound 3
[0274] In a 500ml three-necked flask, add 8g (1.0eq) compound 1, 12.7g (2.0eq) 2,4-dichloro-5-trifluoromethylpyrimidine, 6.8g (1.7eq) aluminum trichloride, 400mlDCE, nitrogen protection React overnight at 80°C. TLC monitored the reaction to complete. Add water to quench the reaction, separate the aqueous phase and add EA to extract, combin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com