Electrolyte, preparation method thereof and lithium ion battery
A lithium-ion battery and electrolyte technology, applied in secondary batteries, secondary battery repair/maintenance, circuits, etc., can solve the problems of electrolyte cycle performance, high temperature performance, room temperature and low temperature power performance, etc. The effect of stability, simple operation and short process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
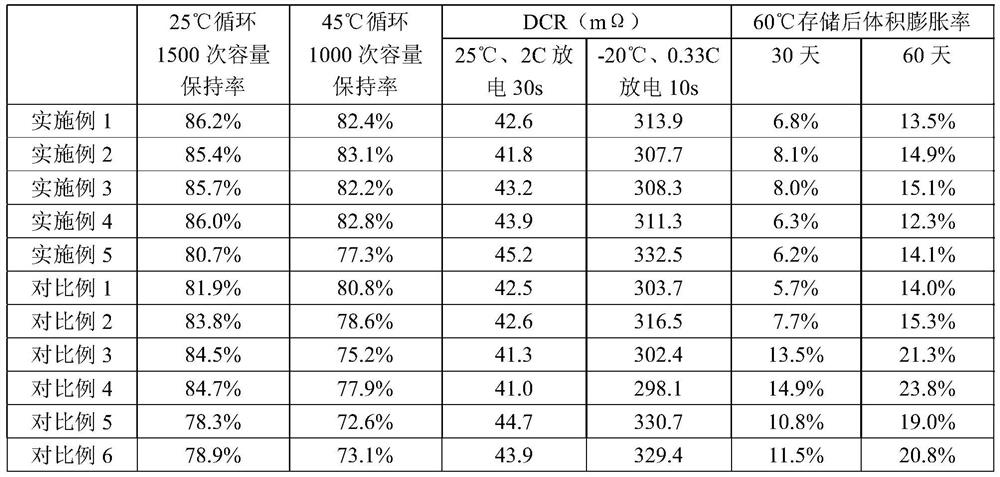
[0062] This embodiment provides an electrolyte, which is composed of an organic solvent, electrolyte salt and additives. The additives are unsaturated carbonate additives, lithium salt additives, additive A and additive B.
[0063] The unsaturated carbonate additive is vinylene carbonate, with a mass fraction of 0.8% in the electrolyte;
[0064] The lithium salt additive is lithium bisfluorosulfonimide, with a mass fraction of 2% in the electrolyte;
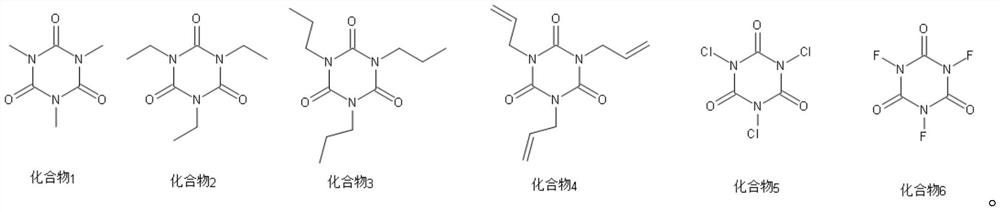
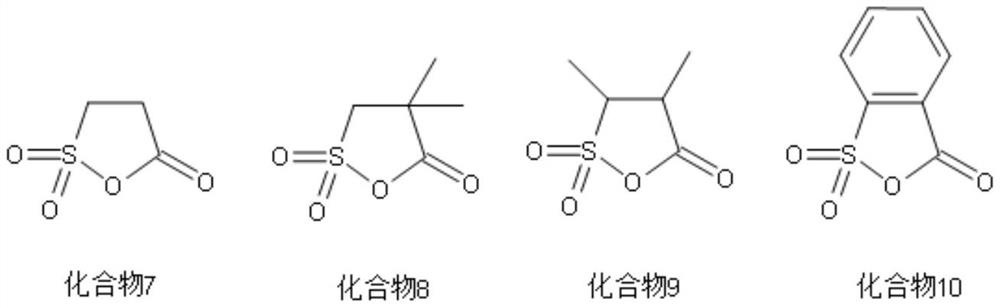
[0065] Additive A is 2-sulfobenzoic anhydride (compound 10), the mass fraction in the electrolyte is 0.6%;
[0066] Additive B is trimethyl isocyanurate (compound 1), with a mass fraction of 0.6% in the electrolyte.
[0067] The lithium salt is lithium hexafluorophosphate, and its concentration in the electrolyte is 1.0 mol / L.
[0068] Described organic solvent is made up of ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate and chain carboxylate (ethyl propionate), and the total volume of organic solvent is 100%, ...
Embodiment 2
[0073] This embodiment provides an electrolyte, which is composed of an organic solvent, electrolyte salt and additives. The additives are unsaturated carbonate additives, lithium salt additives, additive A and additive B.
[0074] The unsaturated carbonate additive is fluoroethylene carbonate, with a mass fraction of 0.6% in the electrolyte;
[0075] The lithium salt additive is lithium difluorophosphate, with a mass fraction of 1.0% in the electrolyte;
[0076] Additive A is 2-sulfopropionic anhydride (compound 7), with a mass fraction of 0.8% in the electrolyte;
[0077] Additive B is triethyl isocyanurate (compound 2), with a mass fraction of 0.4% in the electrolyte.
[0078] The lithium salt is lithium hexafluorophosphate, and its concentration in the electrolyte is 1.2 mol / L.
[0079] Described organic solvent is made up of ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate and chain carboxylate (methyl propionate), and the total volume of organic solvent ...
Embodiment 3
[0084] This embodiment provides an electrolyte, which is composed of an organic solvent, electrolyte salt and additives. The additives are unsaturated carbonate additives, lithium salt additives, additive A and additive B.
[0085] The unsaturated carbonate additive is a mixture of vinylene carbonate and fluoroethylene carbonate (the mass ratio of vinylene carbonate and fluoroethylene carbonate is 1:1), and the mass fraction in the electrolyte is 1.0%;
[0086] The lithium salt additive is lithium bisoxalate borate, with a mass fraction of 0.5% in the electrolyte;
[0087] Additive A is 4,4-dimethyl-2-sulfopropionic anhydride (compound 8), the mass fraction in the electrolyte is 1.0%;
[0088] Additive B is trichloroisocyanuric acid (compound 5), with a mass fraction of 1.0% in the electrolyte.
[0089] The lithium salt is lithium hexafluorophosphate, and its concentration in the electrolyte is 1.2 mol / L.
[0090] Described organic solvent is made up of ethylene carbonate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com