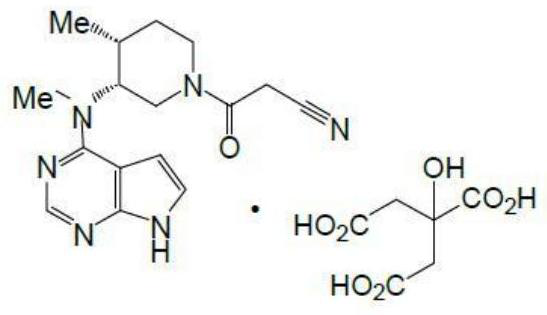
Preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, applied in carboxylate preparation, organic chemistry, etc., can solve the problems of low product yield, difficult control of isomers, expensive and rare raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
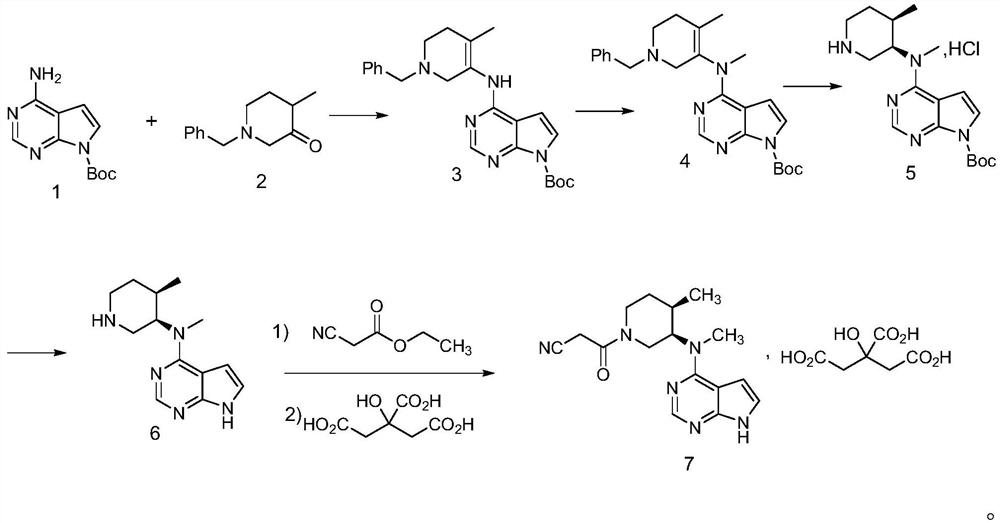
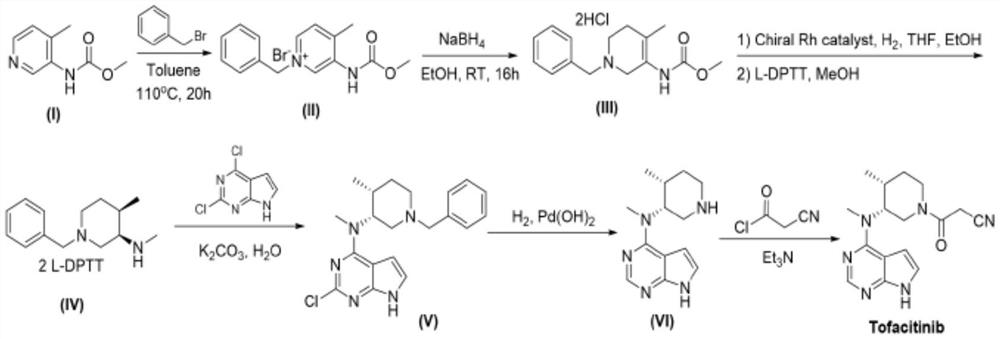
[0044] The following examples provided by the present invention are only to further illustrate the technical solutions and technical effects of the present invention, rather than to limit the present invention.
[0045] 1) Preparation of Intermediate 3
[0046] Weigh 20.0g of compound 1 (90mmol), 18.3g of compound 2 (90mmol), 0.20g of glacial acetic acid and 200ml of dichloromethane, and incubate at 20-30°C for 2 hours, TLC detects that the reaction is complete, and concentrate under reduced pressure to obtain an oily intermediate 3, directly used in the next reaction;
[0047] 2) Preparation of Intermediate 4
[0048] Add 200ml of acetone and 26.3g of potassium carbonate (90mmol) to the above oil, under nitrogen protection, add 13.4g of methyl iodide (94.5mmol) dropwise at 20-30°C, after the addition is complete, keep warm for 3 hours, filter, and concentrate under reduced pressure to obtain an oil The compound is the intermediate 4, which is directly used in the next step ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com