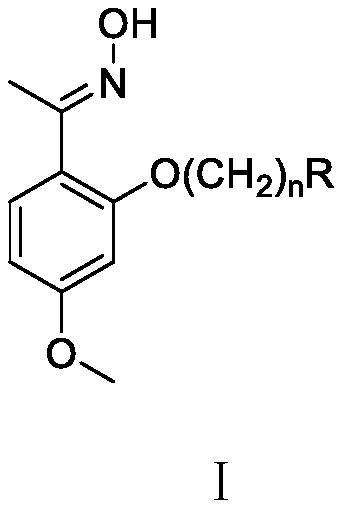
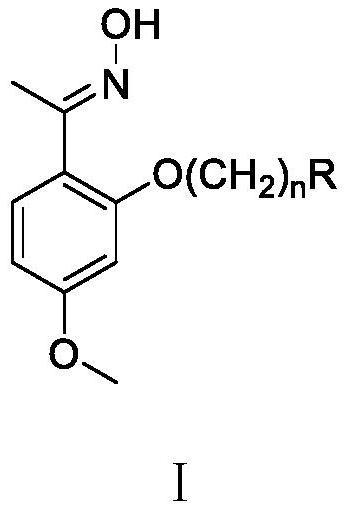
Novel paeonol oxime compound as well as preparation method and medical application thereof
A technology of paeonol oxime and compound, which is applied in the field of new paeonol oxime compound and its preparation, can solve the problems of easy metabolism and poor water solubility, clinical application limitation, easy volatility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
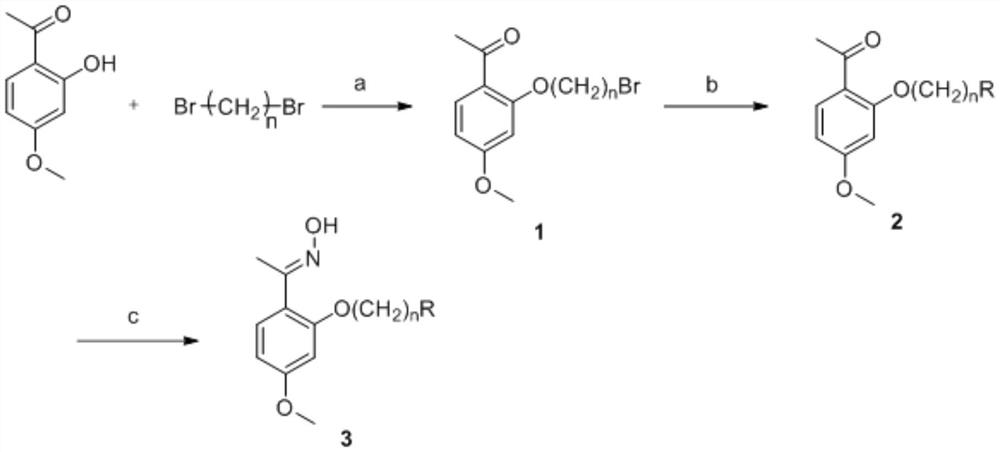
[0117] Preparation of Paeonol Bromohexyl Ether (1)
[0118]Put paeonol (1.66g, 0.01mol), NaOH (2.4g, 0.06mol), 10mL DMF in a 50mL round bottom flask, and stir at room temperature for 20min. Then 1,6-dibromoethane (4.6 mL, 0.03 mol) was added, and the reaction was stirred at 25°C, and the reaction progress was tracked by TLC. After the reaction was completed, the reaction solution was slowly poured into 200 mL of ice water, and extracted with dichloromethane (DCM) (100 mL×3). The organic phase was washed with saturated NaCl solution (100mL×1), anhydrous sodium sulfate (NaCl 2 SO 4 )dry. It was filtered, concentrated, and separated by column chromatography [V (ethyl acetate): V (petroleum ether) = 1: 15] to obtain 2.59 g of a white solid with a yield of 78.7%. m.p.43.0~44.9℃.
[0119] Preparation of 2-[6-(1-morpholine)hexyloxy]paeonol(2)
[0120] Weigh paeonol bromohexyl ether (0.987g, 3mmol) into a 50mL round bottom flask, add 6mL of acetonitrile to dissolve, add dropwise...
Embodiment 2
[0124] Preparation of Paeonol Bromoamyl Ether (1)
[0125] Put paeonol (1.66g, 0.01mol), NaOH (2.4g, 0.06mol), 10mL DMF in a 50mL round bottom flask, and stir at room temperature for 20min. Then 1,5-dibromopentane (4.1 mL, 0.03 mol) was added, the reaction was stirred at 25°C, and the reaction progress was monitored by TLC. After the reaction was completed, the reaction solution was slowly poured into 200 mL of ice water, and extracted with dichloromethane (DCM) (100 mL×3). The organic phase was washed with saturated NaCl solution (100mL×1), anhydrous sodium sulfate (NaCl 2 SO 4 )dry. It was filtered, concentrated, and separated by column chromatography [V (ethyl acetate): V (petroleum ether) = 1: 15] to obtain 2.44 g of a white solid with a yield of 77.5%. m.p.38.0~39.7℃.
[0126] Preparation of 2-[5-(1-piperidine)pentyloxy]paeonol(2)
[0127] Weigh paeonol bromoamyl ether (0.630g, 2mmol) into a 50mL round bottom flask, add 6mL of acetonitrile to dissolve, add piperidin...
Embodiment 3
[0131] Preparation of Paeonol Bromobutyl Ether (1)
[0132] Put paeonol (1.66g, 0.01mol), NaOH (2.4g, 0.06mol), 10mL DMF in a 50mL round bottom flask, and stir at room temperature for 20min. Then 1,4-dibromobutane (3 mL, 0.03 mol) was added, the reaction was stirred at 25°C, and the reaction progress was tracked by TLC. After the reaction was completed, the reaction solution was slowly poured into 200 mL of ice water, and extracted with dichloromethane (DCM) (100 mL×3). The organic phase was washed with saturated NaCl solution (100mL×1), anhydrous sodium sulfate (NaCl 2 SO 4 )dry. It was filtered, concentrated, and separated by column chromatography [V (ethyl acetate): V (petroleum ether) = 1: 15] to obtain 2.35 g of a white solid with a yield of 78.1%. m.p.42.9-44.3°C.
[0133] Preparation of 2-[4-(4-methylpiperazine)butoxy]paeonol(2)
[0134] Weigh paeonol bromobutyl ether (0.602g, 2mmol) in a 50mL round bottom flask, add 6mL of acetonitrile to dissolve, add dropwise 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com