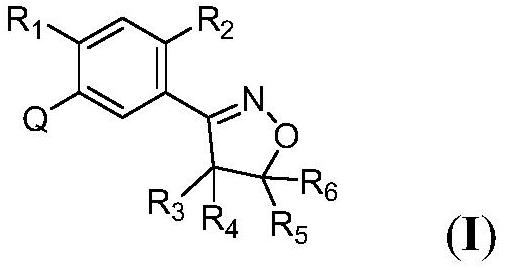
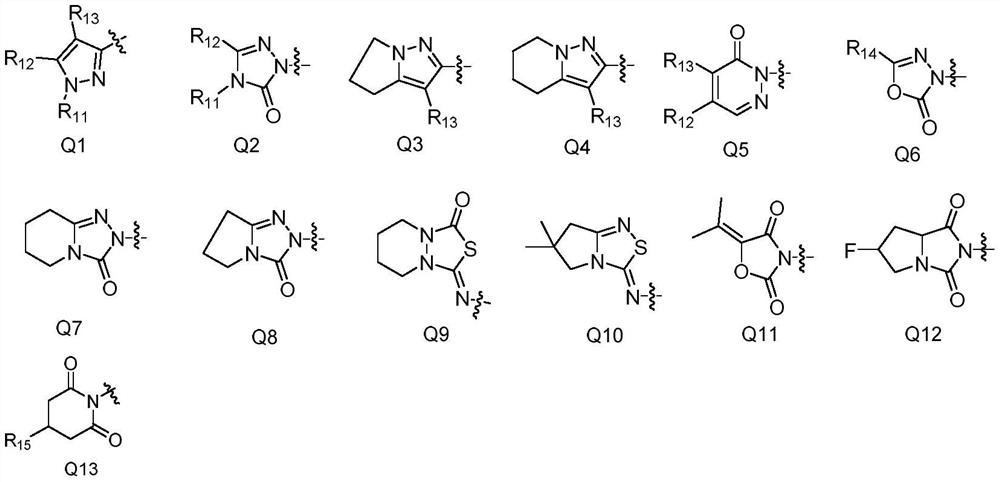
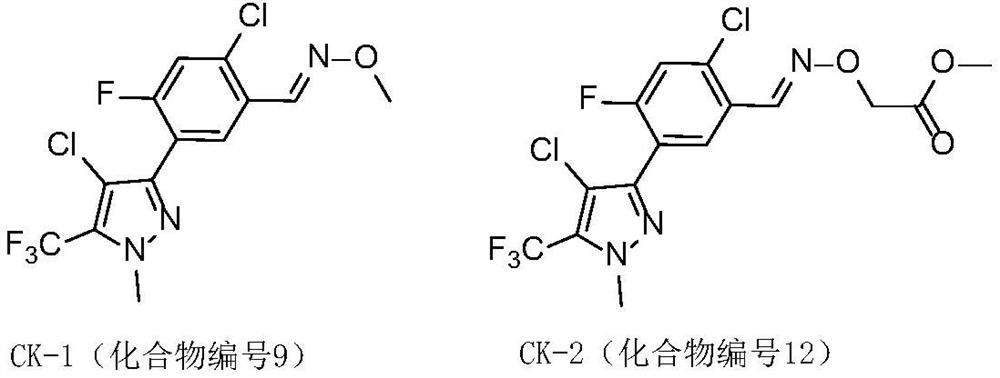
Phenylisoxazoline compound and application thereof
A technology of phenylisoxazoline and compounds, which is applied in the direction of silicon organic compounds, compounds and applications of group 4/14 elements of the periodic table, and can solve problems such as herbicide resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] The preparation of embodiment 1 compound 1-12
[0200]
[0201] 1) Preparation of 2-fluoro-4-chloro-5-methylacetophenone
[0202] Add 22.9g (0.1585mol) of 2-chloro-4-fluoro-toluene and 45.8g (0.344mol) of anhydrous aluminum trichloride to the reaction flask in sequence, and add 18.7g (0.2382mol) of acetyl chloride dropwise while stirring at room temperature, After the dropwise addition was completed for half an hour, the temperature was raised to 50°C to react, and a brown viscous substance was formed. After keeping for 15 hours, it was cooled to room temperature, poured into ice water, extracted with dichloromethane, combined the organic phases, and washed twice with saturated saline. The organic phase was dried over anhydrous magnesium sulfate, filtered and precipitated under reduced pressure, and column chromatography (ethyl acetate:petroleum ether=1:10) obtained 27.4 g of oil.
[0203] 2) Preparation of 1-(4-chloro-2-fluoro-5-methylphenyl)-4,4,4-trifluoroacetyl-...
Embodiment 2
[0230] Embodiment 2 Indoor herbicidal activity assay
[0231] The herbicidal activity testing method of compound of the present invention is as follows:
[0232] Quantitative gramineous weeds (barnyardgrass, golden foxtail) and broad-leaved weeds (zinnia, velvetleum) seeds were respectively sown in paper cups with a diameter of 7 cm filled with nutrient soil, and covered with soil 1 cm after sowing, pressed, After watering, they were cultivated in the greenhouse according to conventional methods. Gramineae weeds grow to 2-3 leaf stage, and broad-leaved weeds 2-4 leaf stage stem and leaf spray treatment; pre-emergence soil spray treatment is carried out 24 hours after sowing. According to the test design dosage, spray treatment (spray pressure 1.95kg / cm 2 , spray volume 500L / hm 2 , track speed 1.48km / h). The experiment was repeated 3 times. After treatment, the test materials are placed in the operating hall. After the medicinal liquid dries naturally, they are placed in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com