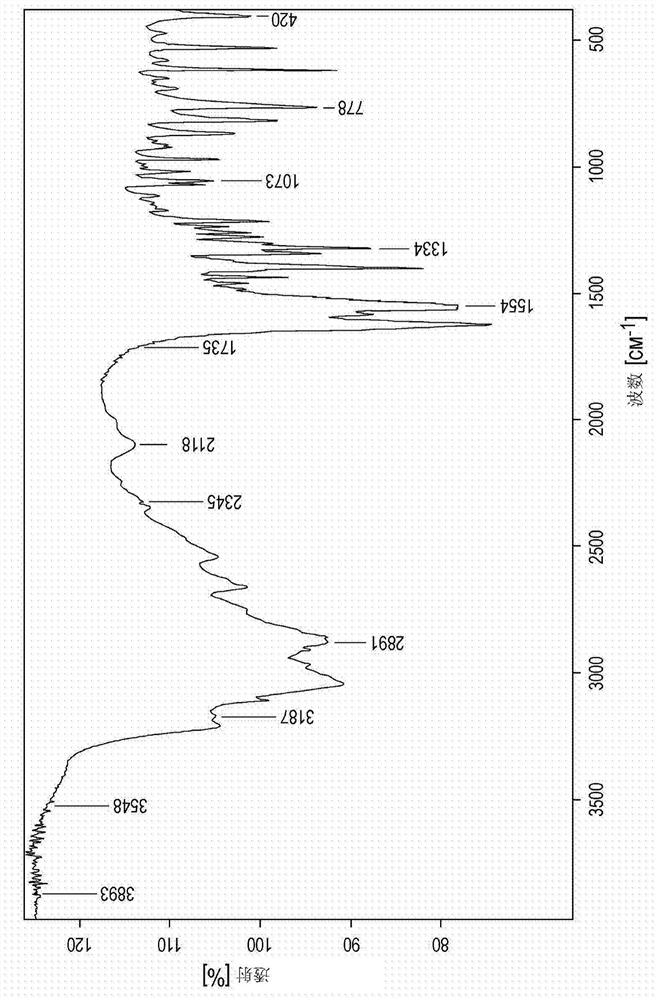
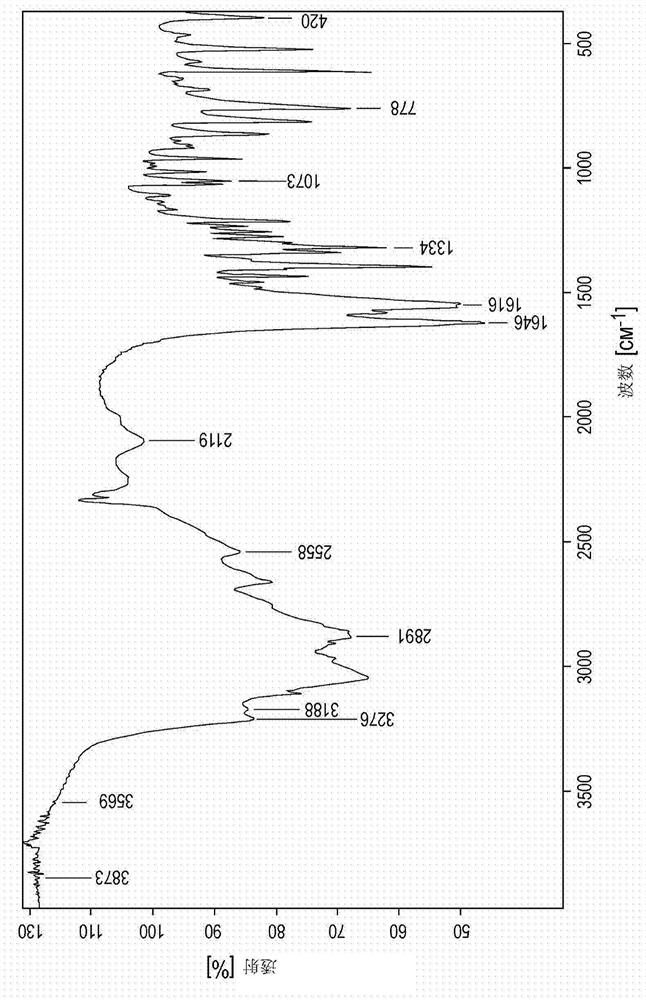
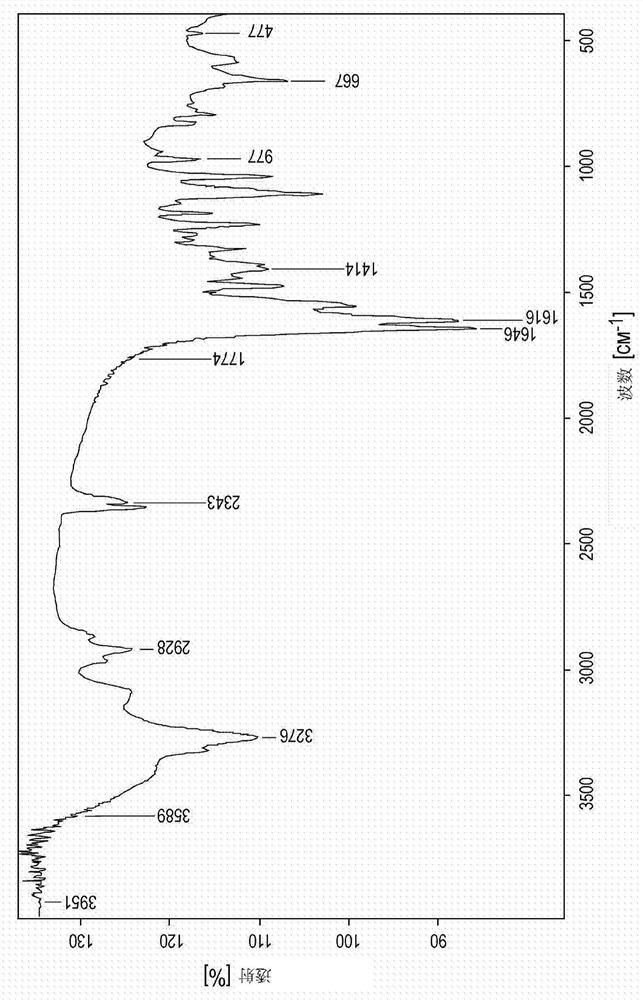
Novel zinc complex, production and use of same
A technology of complexes and uses, applied in the direction of zinc organic compounds, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of ignorance, development and unmet needs for therapeutic uses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1 (not included in the present invention). Mix Zinc Chloride with Gamma-L-Glutamine Histamine
[0144] This example shows that the zinc complex of γ-L-glutamyl histamine cannot be prepared by simple mixing of ingredients such as zinc salt and γ-L-glutamyl histamine, because under these conditions a Mixture of zinc salts and free gamma-L-glutamyl histamine. For example, an aqueous solution of zinc chloride was mixed with γ-L-glutamyl histamine in a ratio of 1:2 and aqueous ammonia was added (until the medium was slightly basic), followed by precipitation of the product resulting in the formation of a white crystalline substance. Elemental analysis data (see Table 1) showed data values close to those calculated for complexed compounds, but the data could not be considered evidence of complex formation. The underestimated amounts of carbon and nitrogen in the samples suggest possible zinc hydroxide formation, which partially loses water during the analysis, can...
Embodiment 2
[0148] Embodiment 2. prepare zinc complex according to the present invention (method No.1)
[0149] In a further study, the authors developed a method for obtaining complexes of zinc with γ-L-glutamyl histamine, which involved the use of aqueous zinc acetate.
[0150] 2.20g (0.01mol) Zn(CH 3 COO) 2 *2H 2 O was dissolved in 20 ml of methanol and added dropwise to 2.40 g (0.01 mol) of γ-L-glutamyl histamine and 1.08 (0.02 mol) of freshly prepared sodium methoxide in 50 ml of methanol under vigorous stirring at room temperature solution in. After adding about half the volume of the zinc acetate solution, the formation of a white precipitate was observed. After all the zinc acetate solution had been added, stirring was continued for about 4 hours, the product was filtered off, washed with water (4 x 10 ml) and dried in vacuo at 65° C. until its weight remained constant. Yield, 2.67 g (88%).
[0151] Two test runs of this technique of the method embodiment were performed and ...
Embodiment 3
[0160] Embodiment 3. according to the present invention obtains zinc complex compound (method No.2)
[0161] The purpose of the modification of the process according to the invention is to improve the filterability of the product obtained and to ensure the most homogeneous distribution of the zinc hydroxide in the reaction mixture.
[0162] For scaling purposes, the issue of distribution uniformity is extremely important for this technique, since the formation of a complex of zinc with γ-L-glutamyl histamine on the zinc hydroxide surface prevents the reaction from proceeding further. To solve this problem, it was proposed to obtain zinc hydroxide in situ by adding sodium hydroxide and zinc acetate solutions simultaneously.
[0163] Synthesis "A": 12.0 g (0.05 mol) of γ-L-glutamyl histamine and 11.0 (0.05 mol) solution of zinc acetate dihydrate in 80 ml of water. The reaction mixture was stirred at 70°C for 4 hours, cooled to room temperature and left overnight. The precipit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com