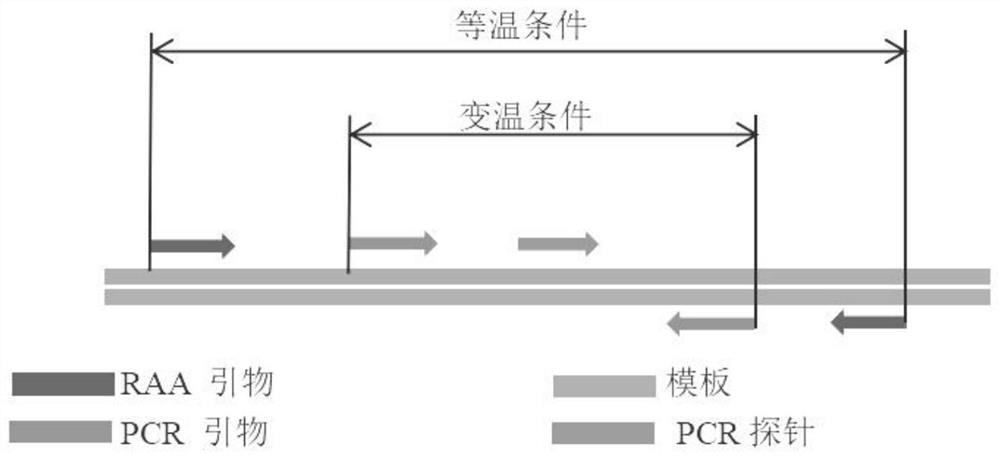
RAP gene detection kit, detection method and application thereof, and RAP virus detection kit
A gene detection and kit technology, applied in the field of gene amplification, can solve the problems of long time-consuming, easy contamination of N-PCR, and difficulty in designing probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
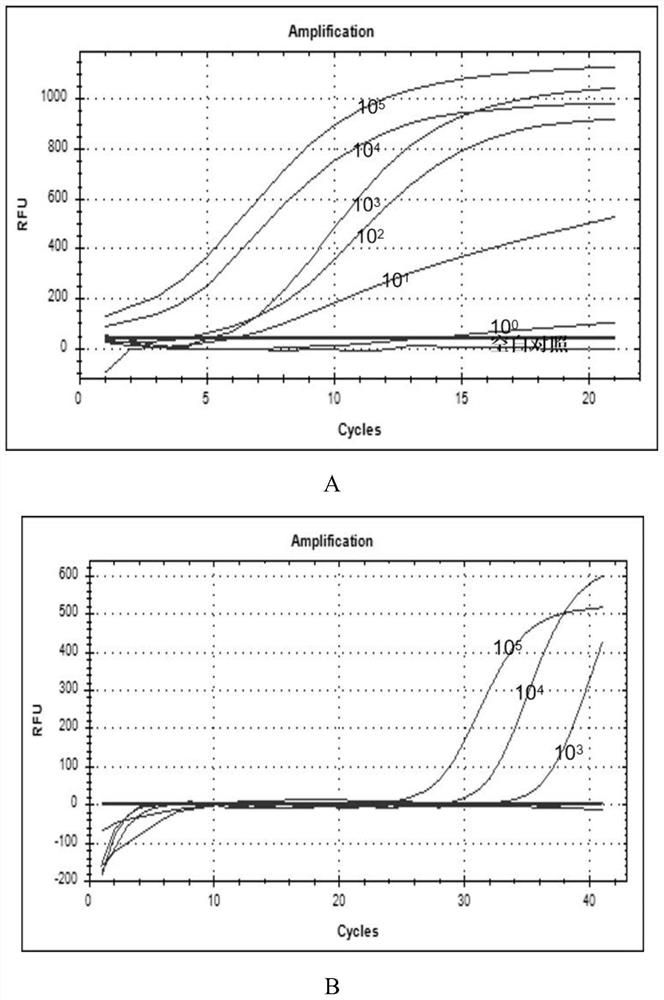
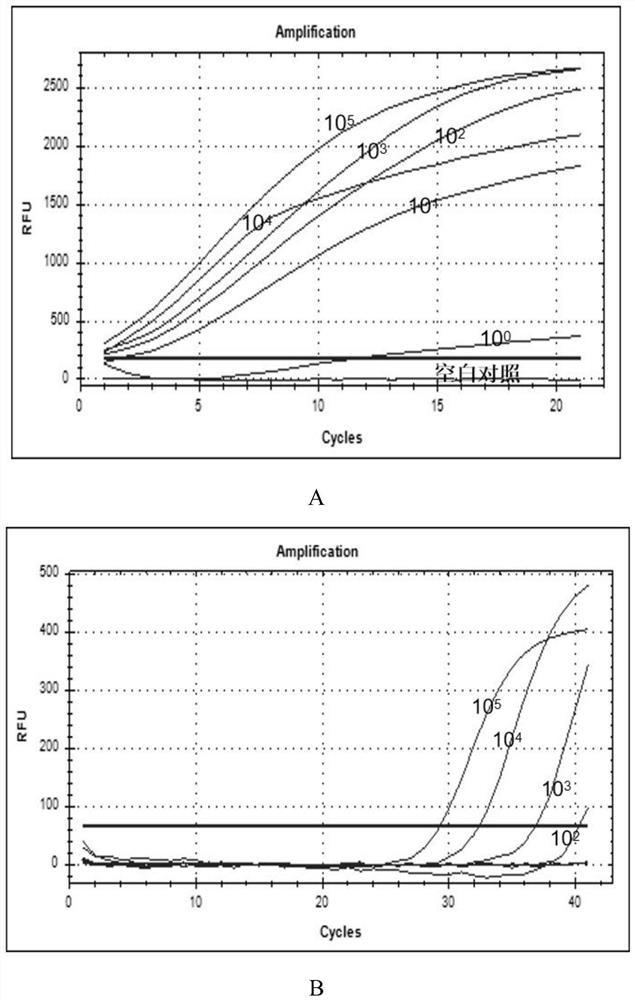
[0047] (50) throat swab specimens from Hebei Provincial Children's Hospital and (403) throat swab specimens and (103) alveolar lavage fluid specimens from Hunan Province CDC.
[0048] The primers and probes of ADV3, ADV7 and RSV in Table 1 were used for detection. Configure the system according to Tables 2 and 3, the final concentration of outer primers is 0.4 μmol / L, and the final concentration of inner primers and probes is 0.2 μmol / L. The RAA kit is the basic method RAA amplification kit of Jiangsu Qitian Co., Ltd., and the PCR kit is the Entrans qPCR Probe Set V2 kit of ABI Company.
[0049] Table 1 Primer and probe information for detection of three viruses
[0050]
[0051]
[0052] Table 2 and 3 are the amplification systems of RAP.
[0053] Table 2 Reaction system of RAA basic kit or RT-RAA basic kit
[0054]
[0055] Table 3 qPCR reaction system
[0056]
[0057]
[0058] Take 10 μl of the reaction system in Table 2 and add it to the cap of the PCR ...
Embodiment 2
[0073] Using the kit provided by the present invention, the method of Example 1 was used to collect 19 virus samples for differential identification. The 19 samples were from the Institute of Viral Disease Prevention and Control, Chinese Center for Disease Control and Prevention. The identification results are shown in Table 7. It can be seen from Table 7 that the RAP method can distinguish and identify the listed samples, and no cross-reaction was observed. The test results show that the method of the present invention can only specifically detect ADV3, ADV7 or RSV, and does not cross-react with other viruses.
[0074] The specific detection analysis of kit described in table 7
[0075] No. sample ADV3 ADV7 RSV 1 Influenza A Negative Negative Negative 2 Influenza B Negative Negative Negative 3 rhinovirus Negative Negative Negative 4 parainfluenza virus Negative Negative Negative 5 human bocavirus Negati...
Embodiment 3
[0077]According to the comparative analysis of Vector11.0 software, specific primers and probes were designed in the conserved regions of ADV3, ADV7 and RSV viruses, and corresponding recombinant plasmids (synthesized by Beijing Qingke Biological Co., Ltd.) were synthesized. 基于ADV3的保守序列Hexon基因,本发明首先提供一条用于检测ADV3的靶序列,其具有SEQ ID NO.16所示的序列(GATAGAGGTCCTAGCTTCAAGCCATATTCCGGCACAGCTTACAATTC ACTCGCTCCTAAGGGCGCGCCCAATACATCTCAGTGGATAGTTACAACA AATGGGGACAATGCAGTAACTACCACCACAAACACATTTGGCATTGCT TCCATGAAGGGAGACAATATTACTAAAGAAGGTTTGCAAATTGGGAAA GACATTACCACTACTGAAGGAGAAGAAAAGCCCATTTATGCCG)或其特异性片段,该序列连接pUC57载体 合成目的质粒;基于ADV7的保守序列Hexon基因,本发明首先提供一条用于检测ADV7的靶序列,其具有SEQ ID NO.17所示的序列(AAGCCATATTCCGGCACAGCTTACAATTCACTGGCTCCTAAGGGCGC GCCTAACACATCTCAGTGGATAGTTACAACGGGAGAAGACAATGCCAC CACATACACATTTGGCATTGCTTCCACGAAGGGAGACAATATTACTAAGGAAGGTTTAGAAATTGGGAAAGACATTACTGCAGACAACAAGCCCATT TATGCCGATAAAACATATCAGCCAGAGCCTCAAGTTGGAGAAGAATCA TGGACTGATATTGATGGAACAAATGAAAAATTTGGAGGTAGAGCTCTTAAACCAGCTACTAAAATGAAGCCATGC)或其特异性片段...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com