Method for fabrication of three-dimensional lung organoid comprising human stem cell-derived alveolar macrophage
A technology of alveolar macrophages and organoids, applied in artificial cell constructs, biochemical equipment and methods, specific-purpose bioreactors/fermenters, etc., can solve problems such as no lung organoid methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: Preparation of three-dimensional lung organoids
[0091] coated board
[0092] The artificial basement membrane matrix that can simulate the extracellular microenvironment (artificial basement membrane-hESC qualified matrix, Cat.#.354277, Corning, USA) was diluted 200 times with cold DMEM / F12 medium (final artificial basement membrane concentration: 250~290μl / 500ml), and put into a 4-well plate (400μl / well), then at 37℃, 5% CO 2 Coat for 1 hour in the incubator.
[0093] Day 1 of differentiation
[0094] Each well of the cell culture plate was coated by adding 10 μg of vitronectin XF (Cat. #.07180, Stem cell technologies) to the plate. By heating at 37°C and 5% CO2 in the presence of TeSR TM -E8 TM Human pluripotent stem cell line H9 (Wicell, WA09-FD) was prepared by culturing in coated plates with culture medium (Cat.#.05940, Stem cell technologies) (2 ml / well). At this time, the medium was changed every day during subculture.
[0095] After r...
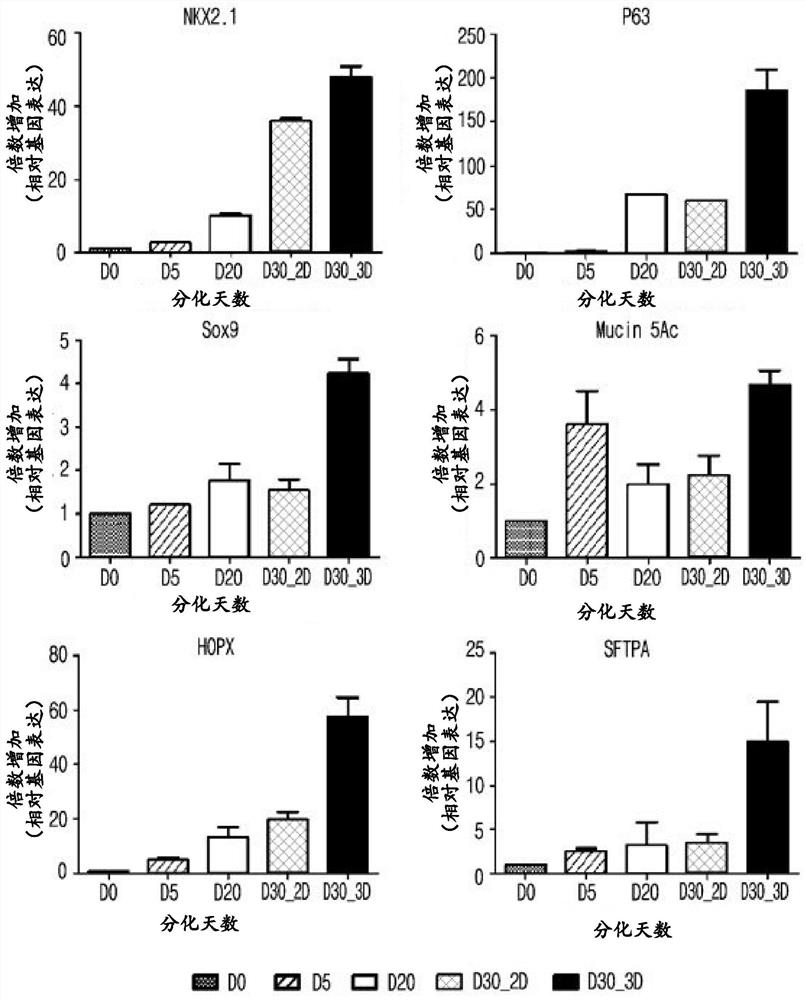
experiment example 1
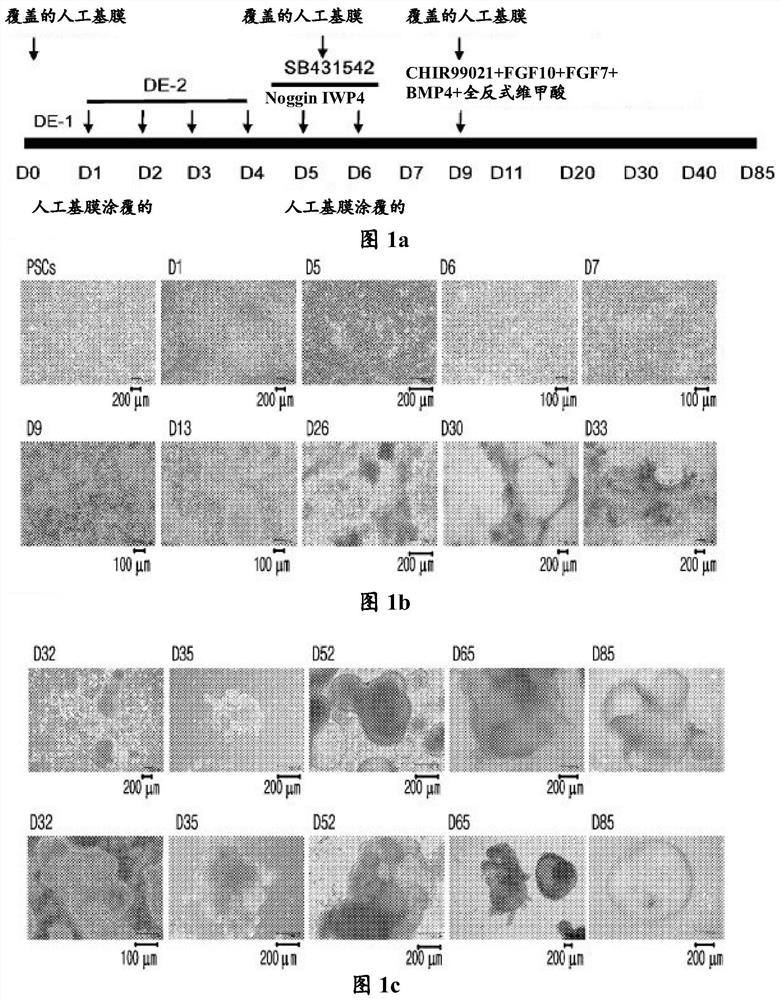
[0120] Experimental example 1: Confirmation of cell morphology in 2D culture and 3D culture after differentiation on day 20
[0121] During the differentiation of hPSCs, the cell morphology was observed by taking pictures while the 2D culture was continued even after the 20th day of differentiation.
[0122] In particular, cells on days 0, 1, 5, 6, 7, 9, and 13 of differentiation, cells on days 26, 30, and 33 of differentiation in which 2D culture was continued, and cells in which differentiation after day 20 were photographed using a microscope Cells at days 32, 35, 52, 65, and 85 of differentiation were changed from two-dimensional culture to three-dimensional culture, and the cell shape was observed.
[0123] As a result, when the cells were differentiated by two-dimensional culture until day 33, tufted cell aggregates were observed, whereas cells differentiated by three-dimensional culture without artificial basement membrane differentiated into clusters similar to actua...
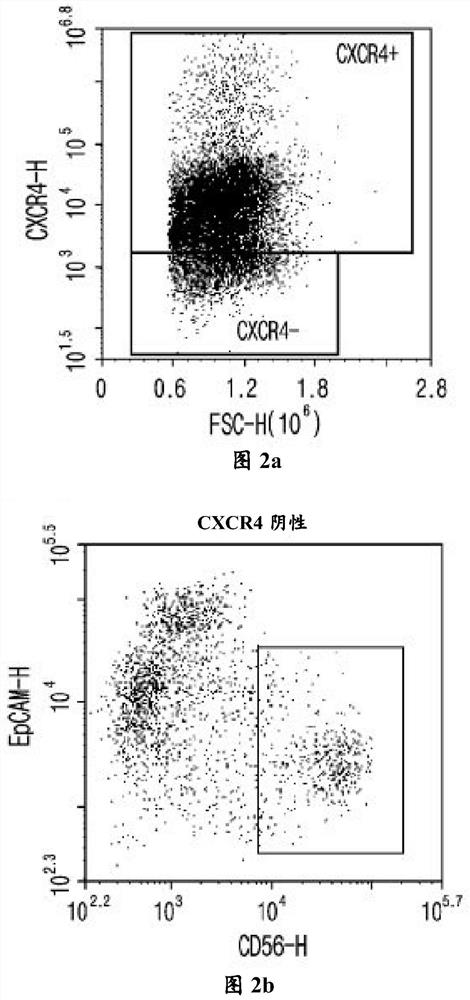
experiment example 2
[0124] Experimental example 2: Confirmation of the expression of CXCR4 markers and mesoderm cell markers on the 4th or 5th day of differentiation
[0125] On day 4 or 5 of differentiation, when the proportion of cells expressing CXCR4 (a complete endoderm (DE) marker) reached 70% to 80%, the cell surface was stained for CXCR4, EpCAM, and CD56 and analyzed by flow cytometry. The degree of differentiation was analyzed technically.
[0126] Specifically, on day 4 or 5 of differentiation, remove the medium of the cells, wash the cells with DPBS, and then add TrypLE TM Express, then incubate for 3 to 5 minutes. After that, remove TrypLE TM Express, and cells were collected by adding DPBS, followed by centrifugation at 1,300 rpm for 5 min. After removing DPBS, cells were suspended in FACS buffer. 2 μl of each antibody (CXCR4, cat.#.551510, BD pharmigen; EpCAM, Cat.#.324204, Biolegend; CD56, Cat.#.555518, BD pharmigen) was added to 1×10 4 ~1×10 5 cells, and react at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com