Cetrorelix preparation and preparation method thereof
A technology of cetrorelix and cetrorelix acetate, which is applied in the field of biomedicine, can solve problems such as poor stability, and achieve the effects of ensuring stability, shortening drying time, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
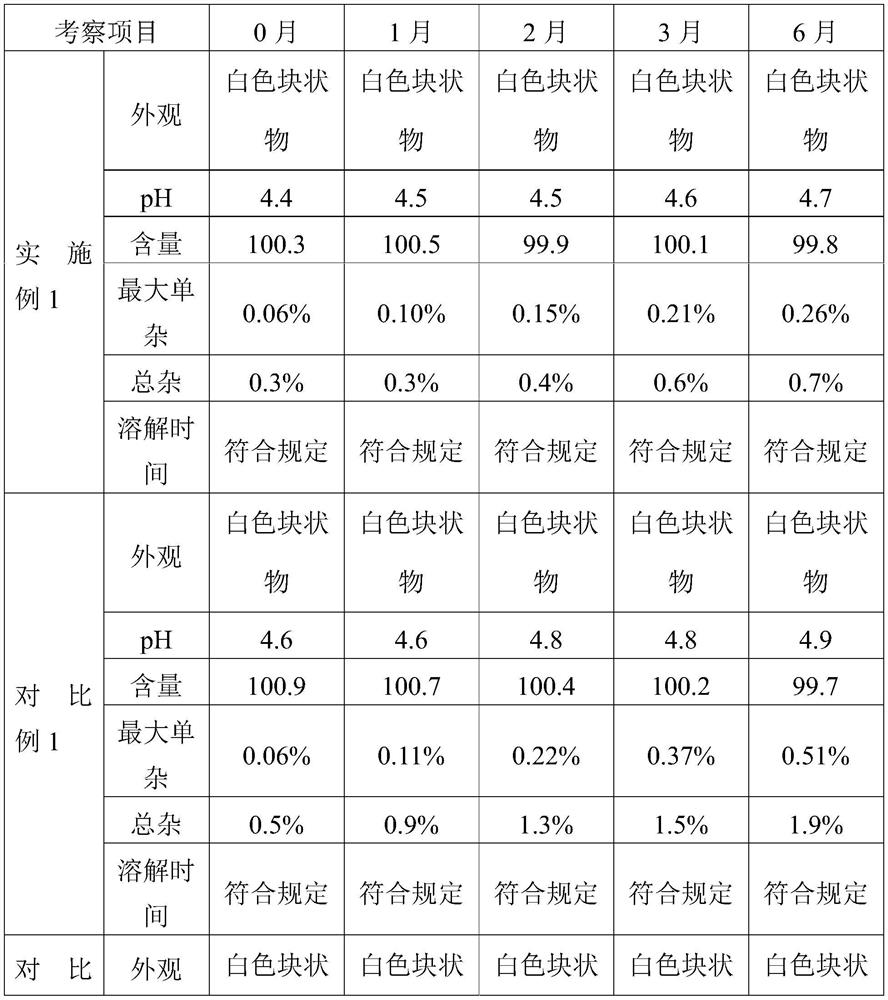
Embodiment 1
[0050] The present invention provides a kind of preparation method of cetrorelix preparation, it comprises the following steps:
[0051] (1) Prepare a mixed solution containing mannitol and cetrorelix acetate:
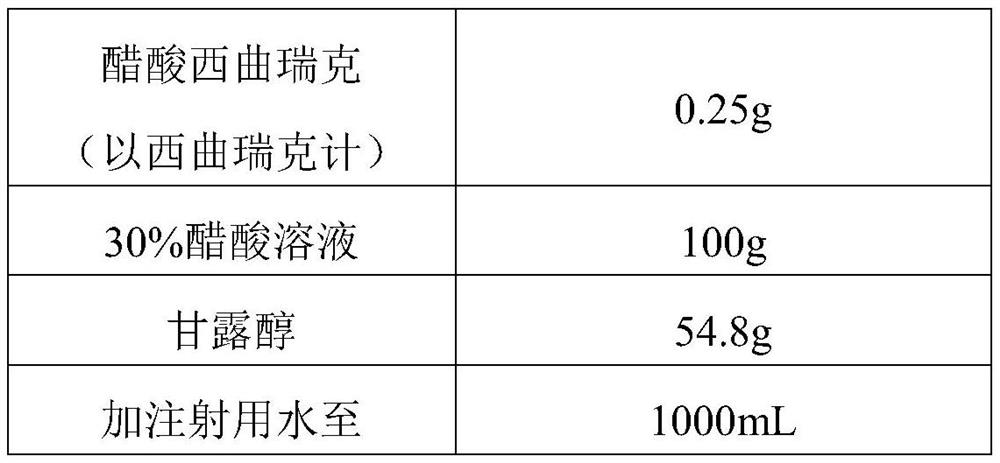
[0052] Table 1 raw material ratio
[0053]
[0054] According to the components shown in Table 1 and the proportioning ratio thereof, raw materials are obtained;
[0055] Add cetrorelix acetate to 30% acetic acid solution (100mL) to dissolve completely, add 600ml water for injection and mix well; then add mannitol to dissolve and mix well; add water for injection to a total volume of 1000mL to obtain Mixed solution of trerelix. The solution was filtered through a 0.2 μm filter.
[0056] (2) freezing:
[0057] Divide the mannitol and cetrorelix acetate acetic acid solution obtained in step (1) into containers (1.0 mL / cartridge), and perform the following steps:
[0058] The first stage: the cooling rate is 0.5°C / min, the temperature of the plate layer is lowered...
Embodiment 2
[0064] The present invention provides a kind of preparation method of cetrorelix preparation, it comprises the following steps:
[0065] (1) Preparation of mannitol, cetrorelix acetate acetic acid solution:
[0066] According to the components shown in Table 1 and the proportioning ratio thereof, raw materials are obtained;
[0067] Add cetrorelix acetate to 30% acetic acid solution (100mL) to dissolve completely, then add 600mL water for injection and mix well; then add mannitol to dissolve and mix well; add water for injection to a total volume of 1000mL to obtain Mixed solution of cetrorelix. The solution was filtered through a 0.2 μm filter.
[0068] (2) freezing:
[0069] Divide the obtained mannitol and cetrorelix acetate acetic acid solution (1.0mL / cartridge) into containers, and perform the following steps:
[0070] The first stage: the cooling rate is 0.5°C / min, the temperature of the plate layer is lowered to -40°C, and the holding time is 1 hour;
[0071] The s...
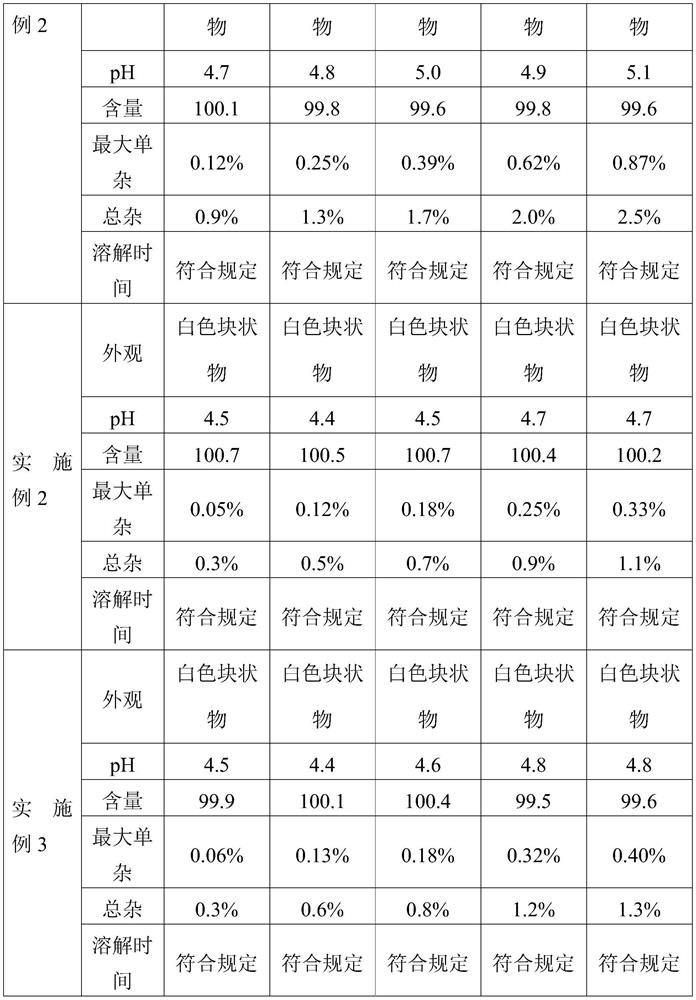
Embodiment 3
[0076] The present invention provides a kind of preparation method of cetrorelix preparation, it comprises the following steps:
[0077] (1) Preparation of mannitol, cetrorelix acetate acetic acid solution:
[0078] According to the components shown in Table 1 and the proportioning ratio thereof, raw materials are obtained;
[0079] Add cetrorelix acetate to 30% acetic acid solution (100mL) to dissolve completely, add 600mL water for injection and mix well; then add mannitol to dissolve and mix well; add water for injection to a total volume of 1000mL to obtain Mixed solution of trerelix. The solution was filtered through a 0.2 μm filter.
[0080] (2) freezing:
[0081] Divide the obtained mannitol and cetrorelix acetate acetic acid solution (1.0mL / cartridge) into containers, and perform the following steps:
[0082] The first stage: the cooling rate is 1°C / min, the temperature of the plate layer is lowered to -30°C, and the holding time is 1 hour;
[0083] The second sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com