Preparation method and application of novel two-dimensional structure copper metal complex
A two-dimensional structure, copper complex technology, applied in the direction of active ingredients of heavy metal compounds, copper organic compounds, 1/11 group organic compounds without C-metal bonds, etc., can solve problems such as limiting clinical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
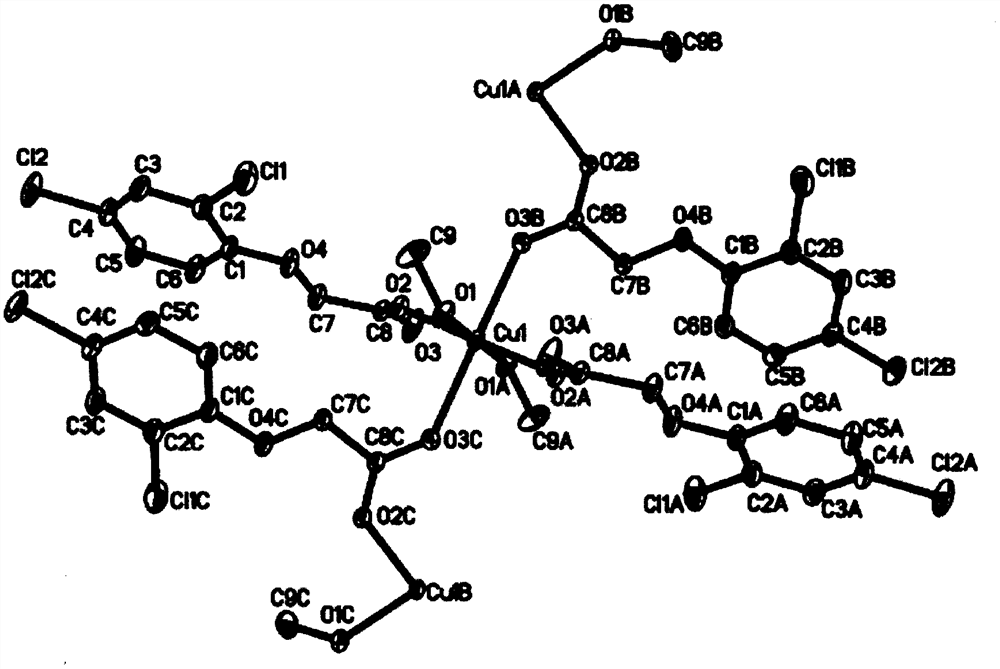
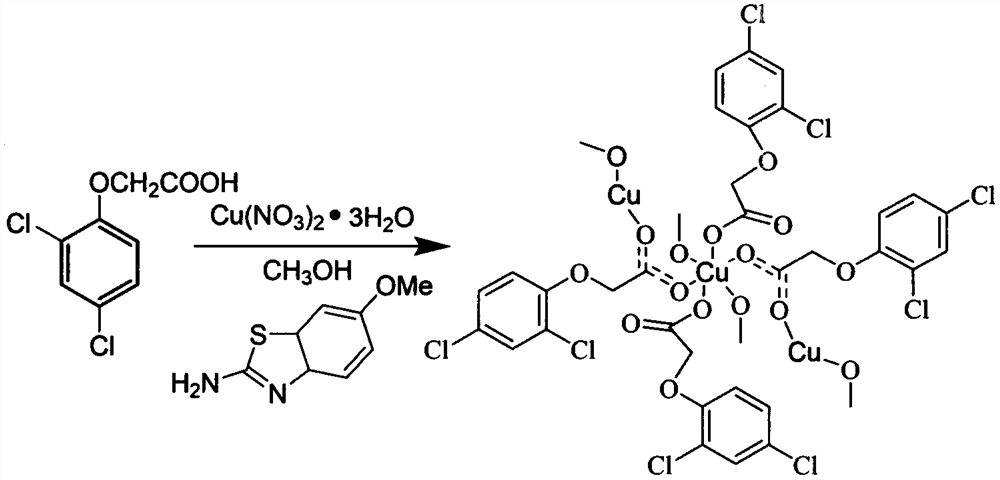
[0029]The present invention also provides embodiments of the preparation method of the new two-dimensional structure copper complex, including the steps of: 2-4-dichlorophenoxycetic acid, 2-amino-6-methoxybenzothiazole in solvent The mixed solution, the copper nitrate was also dissolved in the solvent, stirred, and the copper nitrate solution droplets were added to the mixed solution, and the solution was yellow and green, and the room temperature was allowed to stand at room temperature to obtain a light blue block crystal.
[0030]In order to sufficiently react with the ligand feed, the target complex is preferably obtained, preferably the molar ratio of 2,4-dichlorobenzoxoacetic acid, 2-amino-6-methoxybenzothiazole, and three hydroxisitrate copies of 4-5 : 1-2:1.
[0031]In the present invention, as is preferred, the dripping rate of the copper nitrate solution is 1.0-1.5 ml / min, and the time the reaction is reacted at room temperature for 20-60 min. The standing time is 5-10 days at...
Embodiment 1
[0036]Synthesis of complex: 2,4-dichlorobenzoxic acid (0.40 mmol) and 2-amino-6-methoxybenzothiazole (0.10 mmol) were dissolved in 5 ml of methanol, and three sulfur glycol (0.10 mmol) With 10 ml of methanol, stirring, the copper-nitrate solution was added to a mixed solution at a mixed solution at a mixed solution, and the solution was stirred at room temperature for 20 min, and the pale blue block crystal was precipitated at room temperature. .
Embodiment 2
[0038]Synthesis of complex: 2,4-dichlorobenzoxic acid (0.45 mmol) and 2-amino-6-methoxybenzothiazole (0.15 mmol) were dissolved in 5 ml of methanol, three-squeezable (0.10 mmol) With 10 ml of methanol, stirring, a copper-nitrate solution was added to a mixed solution at 1.2 ml / min, and the solution was yellow and green, and the reaction was stirred at room temperature for 40 min, and at room temperature for about 7 days, precipitated light blue block crystals .
PUM
| Property | Measurement | Unit |
|---|---|---|
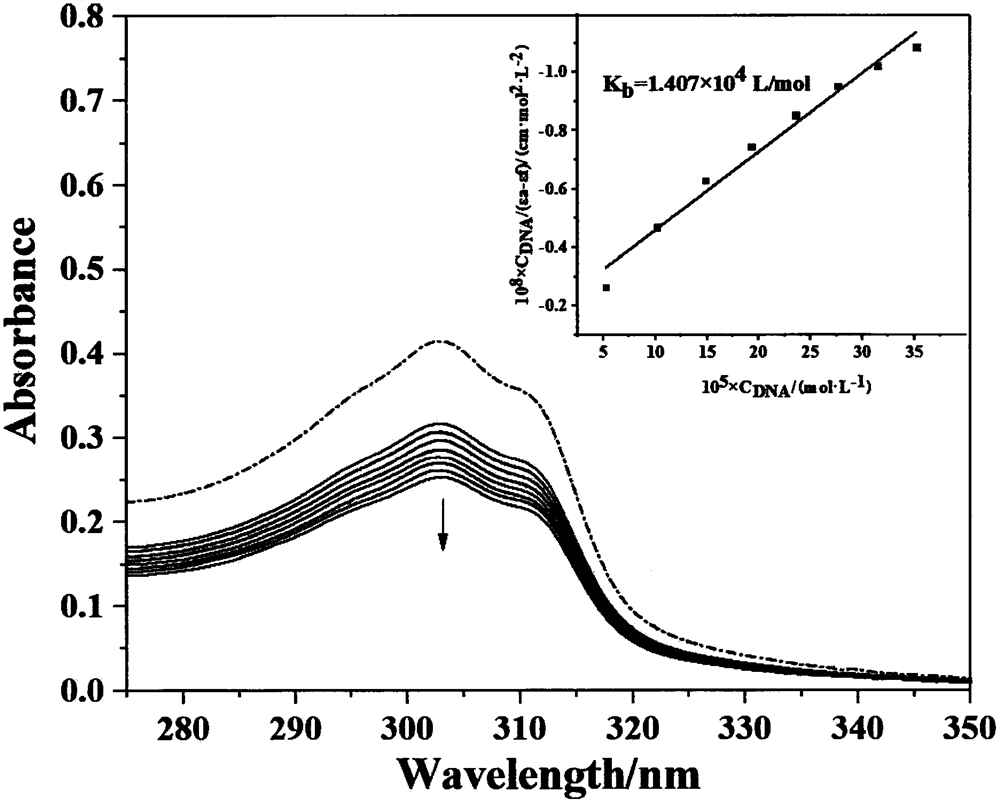
| binding constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com