Novel leukocyte extract mixed factor and preparation method thereof
A technology of mixing factors and extracts, which is applied in the direction of drug combinations, pharmaceutical formulas, peptide/protein components, etc., to achieve the effects of accelerating regulation, improving therapeutic effects, and increasing powder output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
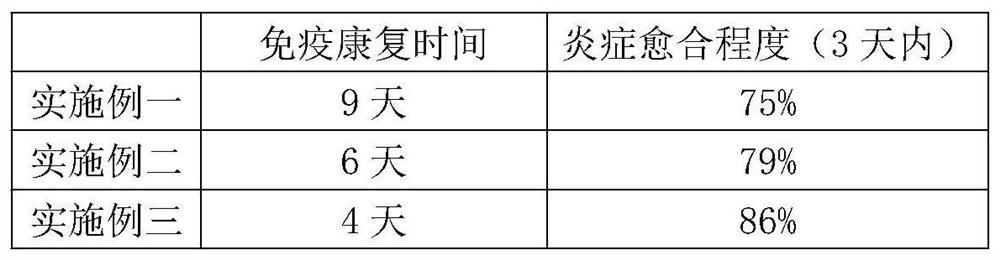
Embodiment 1
[0018] A novel leukocyte extract mixed factor, according to volume percentage, comprises 27 parts of leukocyte extract, 33 parts of platelet lysate, 10 parts of interleukin, 25 parts of stem cell extract, and 5 parts of leukocyte.
[0019] In this example, the selected interleukins are derived from the interleukin-1 family, including IL-1α, IL-1β, IL-1 receptor antagonists, IL-18, IL-36Ra, IL-36α, IL -37, IL-36β, IL-36γ, IL-38 and IL-33, specifically, the mixture of IL-18, IL-36Ra, IL-36α and IL-37 was used in this example.
[0020] In this embodiment, the whitening substances include bisbenzylisoquinoline alkaloids, rubic acid derivatives and synergists; the mass mixing ratio of bisbenzyl isoquinoline alkaloids and rubic acid derivatives is 1:4 .
[0021] In this embodiment, the synergist includes rutabine, anisene, and aminopeptide, and the mass mixing ratio of rutileside, anisene, and aminopeptide is 2:2:3.
[0022] In the present embodiment, the mixed factor in the prese...
Embodiment 2
[0026] A novel leukocyte extract mixed factor, according to volume percentage, comprises 15 parts of leukocyte extract, 25 parts of platelet lysate, 5 parts of interleukin, 22 parts of stem cell extract, and 23 parts of white matter.
[0027] In this example, the selected interleukins are derived from the interleukin-1 family, including IL-1α, IL-1β, IL-1 receptor antagonists, IL-18, IL-36Ra, IL-36α, IL -37, IL-36β, IL-36γ, IL-38 and IL-33, specifically a mixture of IL-18, IL-36Ra, IL-36α, IL-37.
[0028] In this embodiment, the whitening substances include bisbenzylisoquinoline alkaloids, rubic acid derivatives and synergists; the mass mixing ratio of bisbenzyl isoquinoline alkaloids and rubic acid derivatives is 1:4 .
[0029] In this embodiment, the synergist includes rutabine, anisene, and aminopeptide, and the mass mixing ratio of rutileside, anisene, and aminopeptide is 2:2:3.
[0030] In the present embodiment, the mixed factor in the present invention is suitable for...
Embodiment 3
[0034] A novel leukocyte extract mixed factor, according to volume percentage, comprises 25 parts of leukocyte extract, 30 parts of platelet lysate, 7 parts of interleukin, 23 parts of stem cell extract, and 15 parts of white matter.
[0035] In this example, the selected interleukins are derived from the interleukin-1 family, including IL-1α, IL-1β, IL-1 receptor antagonists, IL-18, IL-36Ra, IL-36α, IL -37, IL-36β, IL-36γ, IL-38 and IL-33; specifically a mixture of IL-18, IL-36Ra, IL-36α, IL-37, IL-36β.
[0036] In this embodiment, the whitening substances include bisbenzylisoquinoline alkaloids, rubic acid derivatives and synergists; the mass mixing ratio of bisbenzyl isoquinoline alkaloids and rubic acid derivatives is 1:4 .
[0037] In this embodiment, the synergist includes rutabine, anisene, and aminopeptide, and the mass mixing ratio of rutileside, anisene, and aminopeptide is 2:2:3.
[0038] In the present embodiment, the mixed factor in the present invention is suit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com