Lithium supplement additive for positive electrode of lithium ion capacitor and application thereof
A technology of lithium-ion batteries and additives, which is applied in the field of electrochemical energy storage, and can solve problems such as harsh environmental requirements, difficult control of external short-circuit lithium insertion process, and safety issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
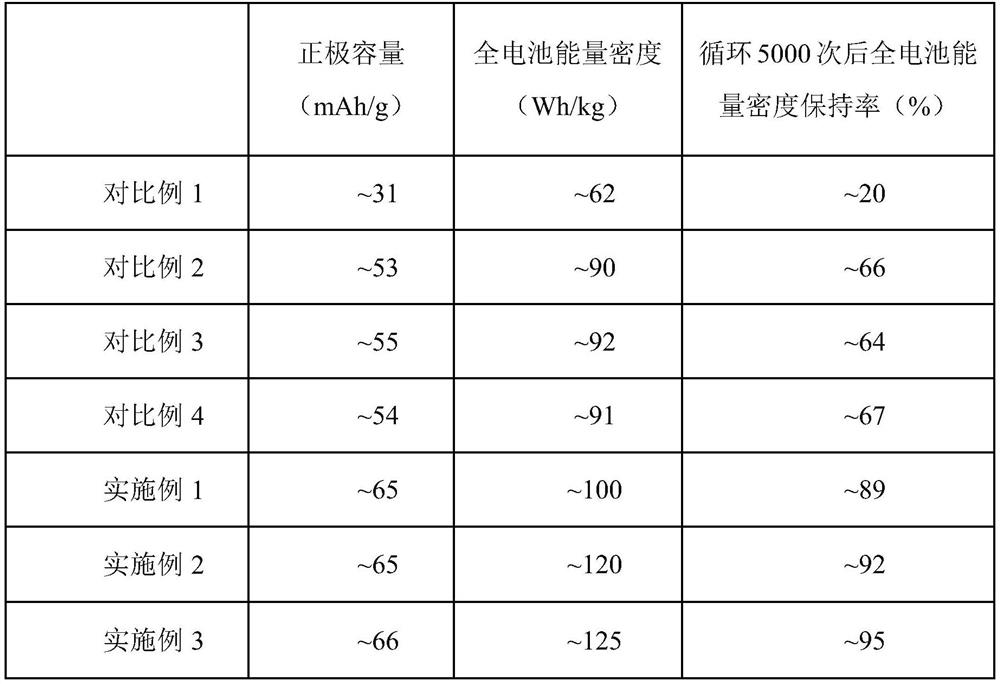
Embodiment 1
[0037] The mass fraction of the configuration is 0.1wt% lithium hydride / ether solution. The activated carbon positive electrode and the hard carbon negative electrode were prepared respectively by the homogenization method. The positive electrode ratio was activated carbon: conductive agent Super P: binder PVDF mass ratio = 8:1:1, and the negative electrode ratio was hard carbon: conductive agent Super P: The mass ratio of binder PVDF=85:9:6. Cut the positive and negative electrodes into 2*2cm-sized electrode sheets, and repeatedly drop-coat the prepared lithium hydride / ether solution on the positive electrode sheet (at this time, the positive electrode is recorded as AC-LiH). The sheet was rolled and compacted, and the amount of lithium hydride was determined by weighing the mass change of the positive electrode sheet (before and after dripping the solution containing lithium hydride), and the mass fraction of lithium hydride in the electrode was 1%. The mass fraction of l...
Embodiment 2
[0039] The mass fraction of the configuration is 2wt% lithium hydride / ether solution. The activated carbon positive electrode and the hard carbon negative electrode were prepared respectively by the homogenization method. The positive electrode ratio was activated carbon: conductive agent Super P: binder PVDF mass ratio = 8:1:1, and the negative electrode ratio was hard carbon: conductive agent Super P: The mass ratio of binder PVDF=85:9:6. Cut the positive and negative electrodes into 2*2cm-sized electrode sheets, and repeatedly drop-coat the prepared lithium hydride / ether solution on the positive electrode sheet (at this time, the positive electrode is recorded as AC-LiH). The sheet is rolled and compacted, and the amount of lithium hydride is determined by weighing the mass change of the positive electrode sheet (before and after dripping the solution containing lithium hydride), and the mass fraction of lithium hydride in the electrode is 2%. Using AC-LiH as the positiv...
Embodiment 3
[0041] The mass fraction of the configuration is 3wt% lithium hydride / ether solution. The activated carbon positive electrode and the hard carbon negative electrode were prepared respectively by the homogenization method. The positive electrode ratio was activated carbon: conductive agent Super P: binder PVDF mass ratio = 8:1:1, and the negative electrode ratio was hard carbon: conductive agent Super P: The mass ratio of binder PVDF=85:9:6. Cut the positive and negative electrodes into 2*2cm-sized electrode sheets, and repeatedly drop-coat the prepared lithium hydride / ether solution on the positive electrode sheet (at this time, the positive electrode is recorded as AC-LiH). The sheet was rolled and compacted, and the amount of lithium hydride was determined by weighing the mass change of the positive electrode sheet (before and after dripping the solution containing lithium hydride), and the proportion of lithium hydride in the electrode was 3%. Using AC-LiH as the positiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com