Preparation and application of glutathione activated photosensitizer
A glutathione and photosensitizer technology, applied in the field of photosensitizer preparation, can solve the problems of strong dependence on oxygen and limit the development of PDT, etc., achieve photosensitivity recovery, excellent endoplasmic reticulum targeting function, and reduce background interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
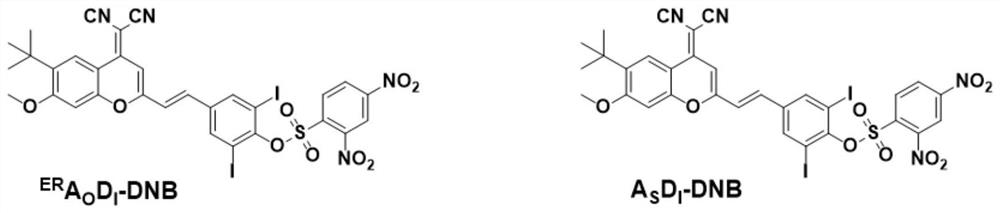
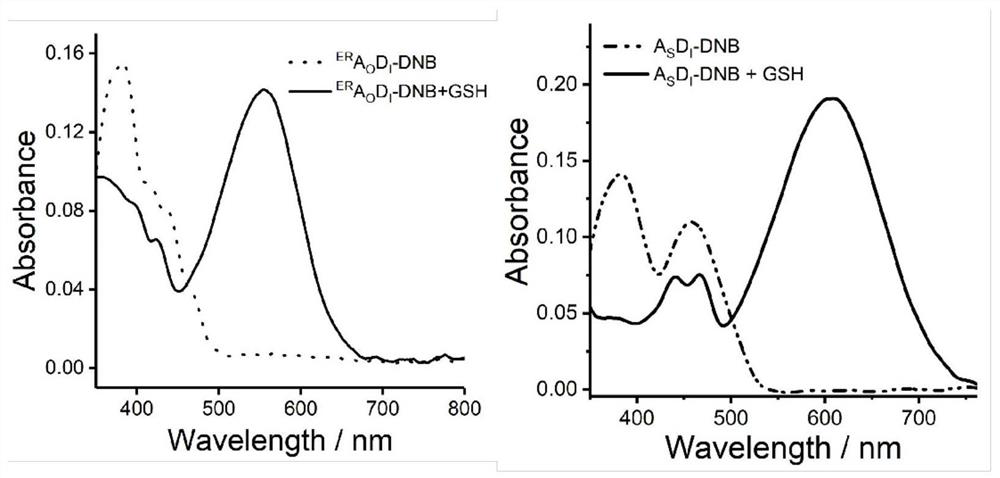
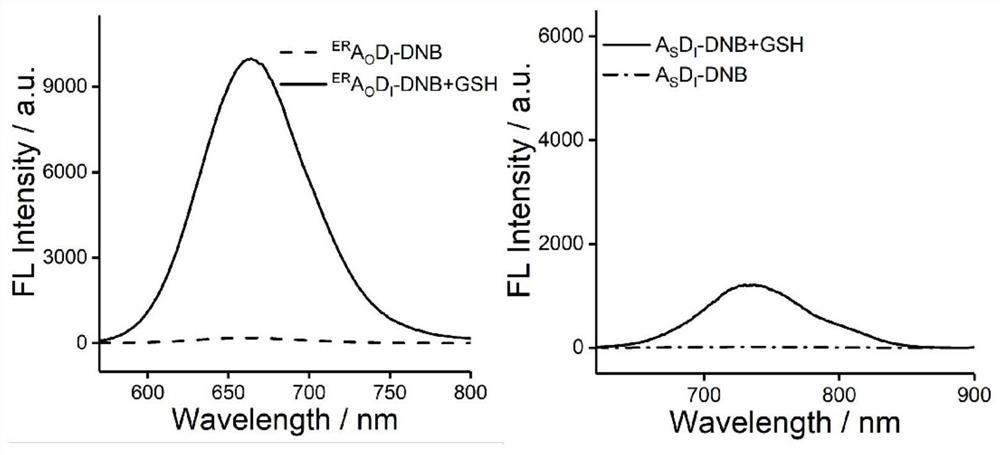
[0023] Embodiment one: synthesis ER A O D. I -DNB
[0024] ER A O D. I (0.325g, 0.50mmol) was dissolved in dichloromethane (10mL), then anhydrous potassium carbonate (0.103g, 0.75mmol) was added, stirred at room temperature for 20min, transferred to an ice-water bath for cooling and stirring for 5min, and then 2,4-Dinitrobenzenesulfonyl chloride (0.20 g, 0.75 mmol) in dichloromethane solution 2 mL was added dropwise under atmosphere protection, the reaction was stirred for 20-30 min in an ice-water bath, and TLC was used to check whether the reaction was complete. After the reaction was completed, the solvent was removed by a vacuum rotary evaporator, and purified by column chromatography to obtain a yellow solid compound ER A O D. I - DNB (0.28 g, 63.6%). 1 H NMR (400MHz, DMSO-d6,) δ (ppm) 9.15 (d, J = 2.3Hz, 1H), 8.72 (dd, J = 8.7, 2.3Hz, 1H), 8.64 (s, 1H), 8.51 (d ,J=8.7Hz,1H),8.29(s,2H),7.68–7.52(m,2H),7.25(s,1H),6.93(s,1H),4.02(s,3H),1.39(s, 9H). 13 C NMR (100...
Embodiment 2
[0025] Embodiment two: synthetic A S D. I -DNB
[0026] A S D. I (250mg, 0.39mmol) was dissolved in dichloromethane (5mL), then Et 3 N 0.78ml, stirred for 10min under ice-water bath conditions, then added dropwise 3mL of a dichloromethane solution of 2,4-dinitrobenzenesulfonyl chloride (125mg, 0.48mmol), the reaction was stirred for 30min under ice-water bath conditions, and then Stir for another 3h. The reaction was checked for completion by TLC. After the reaction was completed, the solvent was removed by a vacuum rotary evaporator, and purified by column chromatography to obtain a red solid compound A S D. I - DNB (231 mg, 68.3%). 1 H NMR (400MHz, CDCl 3 )δ(ppm)8.93(s,1H),8.78(s,1H),8.62(d,J=8.8Hz,1H),8.46(d,J=9.1Hz,1H),8.02(s,2H), 7.72(d, J=8.5Hz, 1H), 7.68–7.53(m, 2H), 7.07(q, J=16.0Hz, 2H), 1.41(s, 9H). 13 C NMR (100MHz, CDCl 3 )δ(ppm)156.00,152.67,152.25,145.06, 141.94,139.32,137.36,133.78,133.65,131.30,131.17,131.03,130.45,129.01,127.19,126.88, 125.26,125.04,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com